Clinical research for life-threatening illnesses requiring emergency hospitalisation: a critical interpretive synthesis of qualitative data related to the experience of participants and their caregivers
- PMID: 36849961
- PMCID: PMC9972707
- DOI: 10.1186/s13063-023-07183-6
Clinical research for life-threatening illnesses requiring emergency hospitalisation: a critical interpretive synthesis of qualitative data related to the experience of participants and their caregivers
Abstract
Background: Research into life-threatening illnesses which require emergency hospitalisation is essential. This group of patients is unique in that they are experiencing an unfolding emergency when they are approached, enrolled, and followed up in a research study. We aimed to synthesise qualitative data from trial participants and surrogate decision-makers to deepen our understanding and inform the design and conduct of future clinical trials for life-threatening illnesses.
Methods: We conducted a critical interpretive synthesis of qualitative data from trial participants and surrogate decision-makers related to the experience of participating in a clinical research study when suffering from a life-threatening illness. A scoping review informed a systematic review of published data. We searched research databases and reviewed papers for inclusion. Primary data and interpretations of data were extracted from each paper. Data were analysed using reciprocal translational analysis, refutational synthesis, and lines of argument synthesis to develop a synthetic construct.
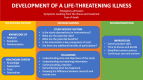
Results: Twenty-two papers were included. Most individuals had no previous knowledge or experience with clinical research. Individuals making decisions were directly experiencing or witness to an unfolding emergency which came with a myriad of physical and psychological symptoms. It was difficult to differentiate clinical research and routine care, and understanding of core concepts around research, particularly randomisation and equipoise, was limited. We found that this led to an underestimation of risk, an overestimation of benefit, and an expectation of being allocated to the intervention arm. The decision-making process was heavily influenced by trust in the research team. Individuals suggested that abbreviated information, presented in different ways and continuously throughout the research process, would have increased knowledge and satisfaction with the research process.
Conclusion: Individuals suffering from a life-threatening illness who are being invited to participate in clinical research need to be managed in a way that adapts to the severity of their illness and there is a need to tailor research processes, including informed consent, accordingly. We provide suggestions for further research and implementation work around research participation for individuals suffering from a life-threatening illness.
Trial registration: PROSPERO CRD42020207296.
Keywords: Clinical research; Clinical trial; Decision-making; Emergency; Informed consent; Qualitative research; Review.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- European Medicines Agency. Guidance for good clinical practice E6 (R2). London: European Medicines Agency; 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guidelin....
-
- Corneli AL, McKenna K, Perry B, Ahmed K, Agot K, Malamatsho F, et al. The science of being a study participant: FEM-PrEP participants’ explanations for overreporting adherence to the study pills and for the whereabouts of unused pills. J Acquir Immune Defic Syndr (1999). 2015;68(5):578–84. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical