Rapid remodeling of the soil lipidome in response to a drying-rewetting event
- PMID: 36849975
- PMCID: PMC9969633
- DOI: 10.1186/s40168-022-01427-4
Rapid remodeling of the soil lipidome in response to a drying-rewetting event
Abstract
Background: Microbiomes contribute to multiple ecosystem services by transforming organic matter in the soil. Extreme shifts in the environment, such as drying-rewetting cycles during drought, can impact the microbial metabolism of organic matter by altering microbial physiology and function. These physiological responses are mediated in part by lipids that are responsible for regulating interactions between cells and the environment. Despite this critical role in regulating the microbial response to stress, little is known about microbial lipids and metabolites in the soil or how they influence phenotypes that are expressed under drying-rewetting cycles. To address this knowledge gap, we conducted a soil incubation experiment to simulate soil drying during a summer drought of an arid grassland, then measured the response of the soil lipidome and metabolome during the first 3 h after wet-up.
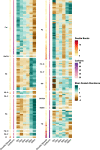
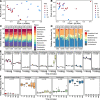
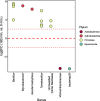
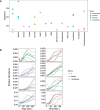
Results: Reduced nutrient access during soil drying incurred a replacement of membrane phospholipids, resulting in a diminished abundance of multiple phosphorus-rich membrane lipids. The hot and dry conditions increased the prevalence of sphingolipids and lipids containing long-chain polyunsaturated fatty acids, both of which are associated with heat and osmotic stress-mitigating properties in fungi. This novel finding suggests that lipids commonly present in eukaryotes such as fungi may play a significant role in supporting community resilience displayed by arid land soil microbiomes during drought. As early as 10 min after rewetting dry soil, distinct changes were observed in several lipids that had bacterial signatures including a rapid increase in the abundance of glycerophospholipids with saturated and short fatty acid chains, prototypical of bacterial membrane lipids. Polar metabolites including disaccharides, nucleic acids, organic acids, inositols, and amino acids also increased in abundance upon rewetting. This rapid metabolic reactivation and growth after rewetting coincided with an increase in the relative abundance of firmicutes, suggesting that members of this phylum were positively impacted by rewetting.
Conclusions: Our study revealed specific changes in lipids and metabolites that are indicative of stress adaptation, substrate use, and cellular recovery during soil drying and subsequent rewetting. The drought-induced nutrient limitation was reflected in the lipidome and polar metabolome, both of which rapidly shifted (within hours) upon rewet. Reduced nutrient access in dry soil caused the replacement of glycerophospholipids with phosphorus-free lipids and impeded resource-expensive osmolyte accumulation. Elevated levels of ceramides and lipids with long-chain polyunsaturated fatty acids in dry soil suggest that lipids likely play an important role in the drought tolerance of microbial taxa capable of synthesizing these lipids. An increasing abundance of bacterial glycerophospholipids and triacylglycerols with fatty acids typical of bacteria and polar metabolites suggest a metabolic recovery in representative bacteria once the environmental conditions are conducive for growth. These results underscore the importance of the soil lipidome as a robust indicator of microbial community responses, especially at the short time scales of cell-environment reactions. Video Abstract.
Keywords: Drying-rewetting; Lipidomics; Metabolomics; Soil; Summer drought.
© 2023. © Battelle Memorial Institute.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Teuling AJ. A hot future for European droughts. Nat Clim Chang. 2018;8:364–365. doi: 10.1038/s41558-018-0154-5. - DOI
-
- Classen AT, et al. Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: what lies ahead? Ecosphere. 2015;6:art130. doi: 10.1890/ES15-00217.1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

