Hypericum perforatum L. Nanoemulsion Mitigates Cisplatin-Induced Chemobrain via Reducing Neurobehavioral Alterations, Oxidative Stress, Neuroinflammation, and Apoptosis in Adult Rats
- PMID: 36851034
- PMCID: PMC9961500
- DOI: 10.3390/toxics11020159
Hypericum perforatum L. Nanoemulsion Mitigates Cisplatin-Induced Chemobrain via Reducing Neurobehavioral Alterations, Oxidative Stress, Neuroinflammation, and Apoptosis in Adult Rats
Erratum in
-
Correction: Khalil et al. Hypericum perforatum L. Nanoemulsion Mitigates Cisplatin-Induced Chemobrain via Reducing Neurobehavioral Alterations, Oxidative Stress, Neuroinflammation, and Apoptosis in Adult Rats. Toxics 2023, 11, 159.Toxics. 2025 May 26;13(6):437. doi: 10.3390/toxics13060437. Toxics. 2025. PMID: 40497403 Free PMC article.
Abstract
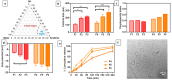
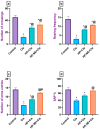
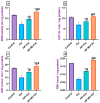
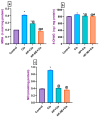
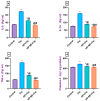
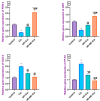
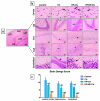
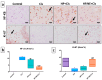
Cisplatin (Cis) is a potent chemotherapeutic agent; however, it is linked with oxidative stress, inflammation, and apoptosis, which may harmfully affect the brain. Hypericum perforatum L. (HP L.) is a strong medicinal plant, but its hydrophobic polyphenolic compounds limit its activity. Therefore, our study aimed to investigate the neuroprotective action of HP L. and its nanoemulsion (NE) against Cis-induced neurotoxicity. The prepared HP.NE was subjected to characterization. The droplet size distribution, surface charge, and morphology were evaluated. In addition, an in vitro dissolution study was conducted. Compared to Cis-intoxicated rats, HP L. and HP.NE-treated rats displayed improved motor activity and spatial working memory. They also showed an increase in their antioxidant defense system and a reduction in the levels of pro-inflammatory cytokines in the brain. Moreover, they showed an increase in the expression levels of the PON-3 and GPX genes, which are associated with a reduction in the brain levels of COX-2 and TP-53. These findings were confirmed by reducing the immunohistochemical expression of nuclear factor kappa (NF-ƘB) and enhanced Ki-67 levels. In conclusion, HP L. is a promising herb and could be used as an adjuvant candidate to ameliorate chemotherapeutic-induced neurotoxicity. Moreover, HP.NE has superior activity in lessening Cis-induced oxidative stress, inflammation, and apoptosis in brain tissue.
Keywords: Hypericum perforatum L.; apoptosis; cisplatin; inflammation; neurobehavior; neuroprotective; oxidative stress; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Correction: Khalil et al. Hypericum perforatum L. Nanoemulsion Mitigates Cisplatin-Induced Chemobrain via Reducing Neurobehavioral Alterations, Oxidative Stress, Neuroinflammation, and Apoptosis in Adult Rats. Toxics 2023, 11, 159.Toxics. 2025 May 26;13(6):437. doi: 10.3390/toxics13060437. Toxics. 2025. PMID: 40497403 Free PMC article.
-
Hypericum perforatum Attenuates Spinal Cord Injury-Induced Oxidative Stress and Apoptosis in the Dorsal Root Ganglion of Rats: Involvement of TRPM2 and TRPV1 Channels.Mol Neurobiol. 2016 Aug;53(6):3540-3551. doi: 10.1007/s12035-015-9292-1. Epub 2015 Jun 23. Mol Neurobiol. 2016. PMID: 26099309
-
Renoprotective effect of Hypericum perforatum against diabetic nephropathy in rats: Insights in the underlying mechanisms.Clin Exp Pharmacol Physiol. 2017 Apr;44(4):509-521. doi: 10.1111/1440-1681.12729. Clin Exp Pharmacol Physiol. 2017. PMID: 28079268
-
Hypericum perforatum: a comprehensive review on pharmacognosy, preclinical studies, putative molecular mechanism, and clinical studies in neurodegenerative diseases.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jun;397(6):3803-3818. doi: 10.1007/s00210-023-02915-6. Epub 2024 Jan 4. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38175276 Review.
-
Hypericum perforatum: pharmacokinetic, mechanism of action, tolerability, and clinical drug-drug interactions.Phytother Res. 2014 May;28(5):643-55. doi: 10.1002/ptr.5050. Epub 2013 Jul 30. Phytother Res. 2014. PMID: 23897801 Review.
Cited by
-
Effect of tangeretin on cisplatin-induced oxido-inflammatory brain damage in rats.J Cell Mol Med. 2024 Jul;28(14):e18565. doi: 10.1111/jcmm.18565. J Cell Mol Med. 2024. PMID: 39044287 Free PMC article.
-
Preparation and characterization of Sorafenib nano-emulsion: impact on pharmacokinetics and toxicity; an in vitro and in vivo study.Drug Deliv Transl Res. 2024 Nov;14(11):3089-3111. doi: 10.1007/s13346-024-01530-z. Epub 2024 Mar 2. Drug Deliv Transl Res. 2024. PMID: 38430357 Free PMC article.
-
Plants' Impact on the Human Brain-Exploring the Neuroprotective and Neurotoxic Potential of Plants.Pharmaceuticals (Basel). 2024 Oct 7;17(10):1339. doi: 10.3390/ph17101339. Pharmaceuticals (Basel). 2024. PMID: 39458980 Free PMC article. Review.
-
Correction: Khalil et al. Hypericum perforatum L. Nanoemulsion Mitigates Cisplatin-Induced Chemobrain via Reducing Neurobehavioral Alterations, Oxidative Stress, Neuroinflammation, and Apoptosis in Adult Rats. Toxics 2023, 11, 159.Toxics. 2025 May 26;13(6):437. doi: 10.3390/toxics13060437. Toxics. 2025. PMID: 40497403 Free PMC article.
-
Neurotrophic Effects of Foeniculum vulgare Ethanol Extracts on Hippocampal Neurons: Role of Anethole in Neurite Outgrowth and Synaptic Development.Int J Mol Sci. 2024 Nov 26;25(23):12701. doi: 10.3390/ijms252312701. Int J Mol Sci. 2024. PMID: 39684414 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

