A Systematic Review and Meta-Analysis on the Real-World Effectiveness of COVID-19 Vaccines against Infection, Symptomatic and Severe COVID-19 Disease Caused by the Omicron Variant (B.1.1.529)
- PMID: 36851102
- PMCID: PMC9965204
- DOI: 10.3390/vaccines11020224
A Systematic Review and Meta-Analysis on the Real-World Effectiveness of COVID-19 Vaccines against Infection, Symptomatic and Severe COVID-19 Disease Caused by the Omicron Variant (B.1.1.529)
Abstract
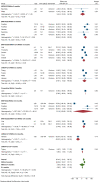
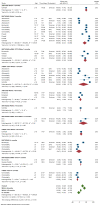
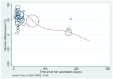
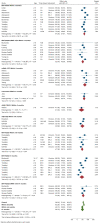
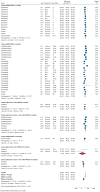
Real-world data on the effectiveness of COVID-19 vaccines against the Omicron variant (B.1.1.529) is limited. This systematic review aimed to investigate the real-world effectiveness and durability of protection conferred by primary course and booster vaccines against confirmed Omicron infection, and severe outcomes. We systematically searched literature up to 1 August 2022. Meta-analysis was performed with the DerSimonian-Laird random-effects model to estimate the pooled vaccine effectiveness (VE). Overall, 28 studies were included representing 11 million individuals. The pooled VE against Omicron infection was 20.4% (95%CI: 12.1-28.7%) and 23.4% (95%CI: 13.5-33.3%) against symptomatic infection with variation based on vaccine type and age groups. VE sharply declined from 28.1% (95%CI: 19.1-37.1%) at three months to 3.9% (95%CI: -24.8-32.7%) at six months. Similar trends were observed for symptomatic Omicron infection. A booster dose restored protection against Omicron infection up to 51.1% (95%CI: 43.8-58.3%) and 57.3% (95%CI: 54.0-60.5%) against symptomatic infection within three months; however, this waned to 32.8% (95%CI: 16.8-48.7%) within six months. VE against severe Omicron infection following the primary course was 63.6% (95%CI: 57.5-69.7%) at three months, decreased to 49% (95%CI: 35.7-63.4%) within six months, and increased to 86% after the first or second booster dose.
Keywords: COVID-19; COVID-19 vaccines; Omicron; booster vaccination; vaccine effectiveness.
Conflict of interest statement
H.S.M. is an investigator on vaccine trials sponsored by the GSK group of companies, Pfizer, Sanofi, and Merck. H.S.M.’s, H.M.’s, B.W.’s, M.M.’s and P.H.A.’s institution receives funding for investigator-led studies from industry, including Pfizer and Sanofi Pasteur; H.S.M., H.M., B.W., M.M. and P.H.A. receive no personal payments from industry. D.D.P.-T. and Z.Y.M.Y. declare no conflict of interest.
Figures



Similar articles
-
Facing the Omicron variant-how well do vaccines protect against mild and severe COVID-19? Third interim analysis of a living systematic review.Front Immunol. 2022 Aug 24;13:940562. doi: 10.3389/fimmu.2022.940562. eCollection 2022. Front Immunol. 2022. PMID: 36091023 Free PMC article.
-
Real-world effectiveness and factors associated with effectiveness of inactivated SARS-CoV-2 vaccines: a systematic review and meta-regression analysis.BMC Med. 2023 Apr 27;21(1):160. doi: 10.1186/s12916-023-02861-3. BMC Med. 2023. PMID: 37106390 Free PMC article.
-
Vaccine effectiveness and duration of protection of COVID-19 mRNA vaccines against Delta and Omicron BA.1 symptomatic and severe COVID-19 outcomes in adults aged 50 years and over in France.Vaccine. 2023 Mar 24;41(13):2280-2288. doi: 10.1016/j.vaccine.2023.02.062. Epub 2023 Feb 27. Vaccine. 2023. PMID: 36870880 Free PMC article.
-
Effectiveness of the booster dose of inactivated COVID-19 vaccine against Omicron BA.5 infection: a matched cohort study of adult close contacts.Respir Res. 2023 Oct 12;24(1):246. doi: 10.1186/s12931-023-02542-y. Respir Res. 2023. PMID: 37828565 Free PMC article.
-
Effectiveness of SARS-CoV-2 vaccines against Omicron infection and severe events: a systematic review and meta-analysis of test-negative design studies.Front Public Health. 2023 Jun 9;11:1195908. doi: 10.3389/fpubh.2023.1195908. eCollection 2023. Front Public Health. 2023. PMID: 37361171 Free PMC article.
Cited by
-
Two centuries of vaccination: historical and conceptual approach and future perspectives.Front Public Health. 2024 Jan 9;11:1326154. doi: 10.3389/fpubh.2023.1326154. eCollection 2023. Front Public Health. 2024. PMID: 38264254 Free PMC article. Review.
-
Key Considerations during the Transition from the Acute Phase of the COVID-19 Pandemic: A Narrative Review.Vaccines (Basel). 2023 Sep 19;11(9):1502. doi: 10.3390/vaccines11091502. Vaccines (Basel). 2023. PMID: 37766178 Free PMC article. Review.
-
Efficacy of adjuvant-associated COVID-19 vaccines against SARS-CoV-2 variants of concern in randomized controlled trials: A systematic review and meta-analysis.Medicine (Baltimore). 2024 Feb 16;103(7):e35201. doi: 10.1097/MD.0000000000035201. Medicine (Baltimore). 2024. PMID: 38363919 Free PMC article.
-
Effectiveness of Adapted COVID-19 Vaccines and Ability to Establish Herd Immunity against Omicron BA.1 and BA4-5 Variants of SARS-CoV-2.Vaccines (Basel). 2023 Dec 10;11(12):1836. doi: 10.3390/vaccines11121836. Vaccines (Basel). 2023. PMID: 38140240 Free PMC article.
-
Severity of Omicron Subvariants and Vaccine Impact in Catalonia, Spain.Vaccines (Basel). 2024 Apr 27;12(5):466. doi: 10.3390/vaccines12050466. Vaccines (Basel). 2024. PMID: 38793717 Free PMC article.
References
-
- World Health Organization [WHO] Tracking SARS-CoV-2 Variants. [(accessed on 2 April 2022)]. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
-
- Bekliz M., Adea K., Vetter P., Eberhardt C.S., Hosszu-Fellous K., Vu D.-L., Puhach O., Essaidi-Laziosi M., Waldvogel-Abramowski S., Stephan C., et al. Neutralization capacity of antibodies elicited through homologous or heterologous infection or vaccination against SARS-CoV-2 VOCs. Nat. Commun. 2022;13:3840. doi: 10.1038/s41467-022-31556-1. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

