Impact of the COVID-19 Pandemic on HPV Vaccinations in Switzerland and Greece: Road to Recovery
- PMID: 36851136
- PMCID: PMC9964352
- DOI: 10.3390/vaccines11020258
Impact of the COVID-19 Pandemic on HPV Vaccinations in Switzerland and Greece: Road to Recovery
Abstract
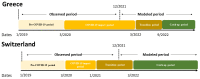
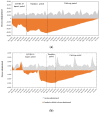
The COVID-19 pandemic has caused significant disruptions to healthcare, including reduced administration of routinely recommended HPV vaccines in a number of European countries. Because the extent and trends of accumulated vaccine dose deficits may vary by country, decision-makers need country-specific information regarding vaccine deficits to plan effective catch-up initiatives. To address this knowledge gap in Switzerland and Greece, this study used a previously published COVID-19 recovery calculator and historical vaccine sales data to quantify the cumulative number of missed doses and the catch-up rate required to clear the deficit in Switzerland and Greece. The resultant cumulative deficit in HPV doses for Switzerland and Greece were 24.4% and 21.7%, respectively, of the total number of doses disseminated in 2019. To clear the dose deficit by December 2025, monthly vaccination rates must be increased by 6.3% and 6.0% compared to 2019 rates in Switzerland and Greece, respectively. This study demonstrates that administration rates of routine HPV vaccines decreased significantly among Swiss and Greek adolescents during the COVID-19 pandemic and that a sustained increase in vaccination rates is necessary to recover the HPV dose deficits identified and to prevent long-term public health consequences.
Keywords: COVID-19; Greece; HPV; Switzerland; adolescents; routine vaccines.
Conflict of interest statement
I.G. and A.S. are employees of MSD, Greece; A.F.-B. is an employee of MSD, Switzerland; S.S. and U.S. are employees of MSD Sweden who may own stock, stock options, or both in Merck & Co., Inc., Rahway, NJ, USA. K.S. is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, who may own stock, stock options, or both in the company. J.W. and H.C. are employees of Adelphi Values PROVE™. Adelphi Values PROVE™ was compensated by MSD for the development of the manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, patents received or pending, or royalties.
Figures

References
-
- Paavonen J., Naud P., Salmerón J., Wheeler C.M., Chow S.-N., Apter D., Kitchener H., Castellsague X., Teixeira J.C., Skinner S.R., et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. - DOI - PubMed
-
- Bonanni P., Faivre P., Lopalco P.L., Joura E., Bergroth T., Varga S., Gemayel N., Drury R. The status of human papillomavirus vaccination recommendation, funding, and coverage in WHO Europe countries (2018–2019) Expert Rev. Vaccines. 2020;19:1073–1083. doi: 10.1080/14760584.2020.1858057. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

