Immunogenicity and SARS-CoV-2 Infection following the Fourth BNT162b2 Booster Dose among Health Care Workers
- PMID: 36851161
- PMCID: PMC9958857
- DOI: 10.3390/vaccines11020283
Immunogenicity and SARS-CoV-2 Infection following the Fourth BNT162b2 Booster Dose among Health Care Workers
Abstract
Introduction: The fourth SARS-CoV-2 vaccine dose was found to protect against infection and more importantly against severe disease and death. It was also shown that the risk of symptomatic or severe disease was related to the antibody levels after vaccination or infection, with lower protection against the BA.4 BA.5 Omicron variants. The aim of our study was to assess the impact of the fourth dose on infection and perception of illness seriousness among healthcare workers (HCWs) at a tertiary health care campus in Haifa, Israel, and to investigate the possible protective effect of antibody levels against infection.
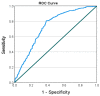
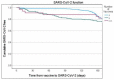
Methods: We conducted a prospective cohort study among fully vaccinated HCWs and retired employees at Rambam Healthcare Campus (RHCC), a tertiary hospital in northern Israel. Participants underwent serial serological tests at 1, 3, 6, 9, 12 and 18 months following the second BNT162b2 vaccine dose. Only a part of the participants chose to receive the fourth vaccine. A multivariable logistic regression was conducted to test the adjusted association between vaccination, and the risk of infection with SARS-CoV-2. Kaplan-Meier SARS-CoV-2 free "survival" analysis was conducted to compare the waning effect of the first and second, third and fourth vaccines. Receiver Operating Characteristic (ROC) curve was plotted for different values of the sixth serology to identify workers at risk for disease.
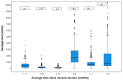
Results: Disease occurrence was more frequent among females, people age 40-50 years old and those with background chronic lung disease. The fourth vaccine was found to have better protection against infection, compared to the third vaccine; however, it also had a faster waning immunity compared to the third vaccine dose. Antibody titer of 955 AU/mL was found as a cutoff protecting from infection.
Conclusions: We found that the fourth vaccine dose had a protective effect, but shorter than the third vaccine dose. Cutoff point of 955 AU/mL was recognized for protection from illness. The decision to vaccinate the population with a booster dose should consider other factors, including the spread of disease at the point, chronic comorbidities and age, especially during shortage of vaccine supply.
Keywords: COVID-19; Immunogenicity; booster; health care workers; serology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kliker L., Zuckerman N., Atari N., Barda N., Gilboa M., Nemet I., Abd Elkader B., Fratty I.S., Jaber H., Mendelson E., et al. COVID-19 vaccination and BA.1 breakthrough infection induce neutralising antibodies which are less efficient against BA.4 and BA.5 Omicron variants, Israel, March to June 2022. Eurosurveillance. 2022;27:2200559. doi: 10.2807/1560-7917.ES.2022.27.30.2200559. - DOI - PMC - PubMed
-
- Ferdinands J.M., Rao S., Dixon B.E., Mitchell P.K., DeSilva M.B., Irving S.A., Lewis N., Natarajan K., Stenehjem E., Grannis S.J., et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: Test negative, case-control study. BMJ. 2022;379:e072141. doi: 10.1136/bmj-2022-072141. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

