Effect of CpG-Oligonucleotide in Enhancing Recombinant Herpes Virus of Turkey-Laryngotracheitis Vaccine-Induced Immune Responses in One-Day-Old Broiler Chickens
- PMID: 36851171
- PMCID: PMC9965839
- DOI: 10.3390/vaccines11020294
Effect of CpG-Oligonucleotide in Enhancing Recombinant Herpes Virus of Turkey-Laryngotracheitis Vaccine-Induced Immune Responses in One-Day-Old Broiler Chickens
Abstract
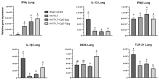
Infectious laryngotracheitis (ILT) is an economically important disease of chickens. While the recombinant vaccines can reduce clinical disease severity, the associated drawbacks are poor immunogenicity and delayed onset of immunity. Here, we used CpG-oligonucleotides (ODN) as an in ovo adjuvant in boosting recombinant herpesvirus of turkey-laryngotracheitis (rHVT-LT) vaccine-induced responses in one-day-old broiler chickens. Two CpG-ODN doses (5 and 10 μg/egg) with no adverse effect on the vaccine-virus replication or chick hatchability were selected for immune-response evaluation. Results showed that while CpG-ODN adjuvantation induced an increased transcription of splenic IFNγ and IL-1β, and lung IFNγ genes, the IL-1β gene expression in the lung was significantly downregulated compared to the control. Additionally, the transcription of toll-like receptor (TLR)21 in the spleen and lung and inducible nitric oxide synthase (iNOS) in the spleen of all vaccinated groups was significantly reduced. Furthermore, splenic cellular immunophenotyping showed that the CpG-ODN-10μg adjuvanted vaccination induced a significantly higher number of macrophages, TCRγδ+, and CD4+ T cells as well as a higher frequency of activated T cells (CD4+CD44+) when compared to the control. Collectively, the findings suggested that CpG-ODN can boost rHVT-LT-induced immune responses in day-old chicks, which may help in anti-ILT defense during their later stages of life.
Keywords: CpG-ODN; adjuvant; chickens; immune response; infectious laryngotracheitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

