Human Papillomavirus Vaccination Uptake in the Rio Grande Valley: Results from a Pilot Community-Based Educational and School-Based Vaccination Program and Its Expansion
- PMID: 36851207
- PMCID: PMC9961922
- DOI: 10.3390/vaccines11020329
Human Papillomavirus Vaccination Uptake in the Rio Grande Valley: Results from a Pilot Community-Based Educational and School-Based Vaccination Program and Its Expansion
Abstract
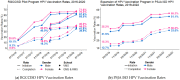
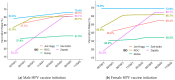
Human papillomavirus (HPV) vaccine is a safe and effective strategy for reducing HPV morbidity and mortality. Schools have become an increasingly attractive setting for delivering vaccinations and supporting vaccination health literacy and decisional support. This study assesses the effectiveness of a community-based, physician-led HPV education campaign (starting in 2016) and onsite middle school-based HPV vaccination program across six school districts (2017, 2019, 2020) in a rural, medically underserved Texas area (Rio Grande Valley). Pre- and post-intervention HPV vaccination rates were tracked against the 2016 National Immunization Survey-Teen target rates (initiation: 49.3%; completion: 32.9%). Summary statistics were stratified by gender, school district, and grade level. The study reached 19,951 students who received HPV vaccines directly or indirectly through our program (10,289 females; 9662 males) (August 2016-August 2022). Of those, 2145 students (1074 females; 1071 males) were vaccinated directly through our program. The overall HPV up-to-date (UTD) rates were 58.8%. The overall median age at HPV vaccine initiation and HPV-UTD (range) was 11 years (9-21) and 12 years (9-20). The overall median interval between HPV vaccine doses (range) was 291 days (146-2968). Recommending HPV vaccine initiation at younger ages increases HPV vaccine completion and providing access to HPV vaccines encourages on-time vaccination and completion.
Keywords: Rio Grande Valley; adolescent health; human papillomavirus vaccine; human papillomavirus-related cancers and diseases; provider recommendation; school-based vaccination.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

