Immune Responses to HBV Vaccine in People Living with HIV (PLWHs) Who Achieved Successful Treatment: A Prospective Cohort Study
- PMID: 36851279
- PMCID: PMC9967144
- DOI: 10.3390/vaccines11020400
Immune Responses to HBV Vaccine in People Living with HIV (PLWHs) Who Achieved Successful Treatment: A Prospective Cohort Study
Abstract
Background: Understanding immune responses after HBV vaccination is important to prevent HBV infection in PLWH and to achieve successful treatment.
Methods: Thirty-two PLWHs with CD4+ cell count > 350 cells/µL and HIV RNA < 200 copies/mL were vaccinated with 20 µg of HBV vaccine at weeks 0, 4, and 24 in this prospective study. We measured total HIV DNA levels, HBsAb titers and HBsAg-specific T-cell responses during follow-up time.
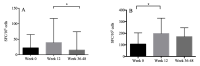
Results: All patients achieved protective HBsAb titer after immunization. The magnitude of the IFN-r and TNF-a response to HBsAg was 22.0 (IQR: 6.5-65.0) and 106.50 (IQR: 58.5-203.0) spot-forming cells (SFC)/105 PBMC, respectively at week 0. The level of IFN-r secreted at weeks 12 and weeks 36 to 48 was comparable with that at week 0. However, IFN-r response was higher at weeks 12 than that at weeks 36 to 48 (p = 0.02). The level of TNF-a secreted at weeks 12 was higher than that at week 0 (p < 0.001). Total HIV DNA levels were 2.76 (IQR: 2.47-3.07), 2.77 (IQR: 2.50-3.09), 2.77 (IQR: 2.41-2.89) log10 copies/106 PBMCs at weeks 0, 12, 36 to 48, respectively. No correlation was observed between IFN-r and TNF-a levels and HBsAb titer as well as total HIV DNA levels after immunization.
Conclusion: Humoral immunity was satisfactory, but cellular immunity and decline in HIV reservoir were not optimal after HBV vaccine immunization in these patients.
Keywords: HBV vaccine; IFN-r; PLWH; TNF-a; total HIV DNA levels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bollinger R.C., Thio C.L., Sulkowski M.S., McKenzie-White J., Thomas D.L., Flexner C. Addressing the global burden of hepatitis B virus while developing long-acting injectables for the prevention and treatment of HIV. Lancet. HIV. 2020;7:e443–e448. doi: 10.1016/S2352-3018(19)30342-X. - DOI - PMC - PubMed
-
- Audsley J., Avihingsanon A., Littlejohn M., Bowden S., Matthews G.V., Fairley C.K., Lewin S.R., Sasadeusz J. Long-Term TDF-Inclusive ART and Progressive Rates of HBsAg Loss in HIV-HBV Coinfection-Lessons for Functional HBV Cure? JAIDS J. Acquir. Immune Defic. Syndr. 2020;84:527–533. doi: 10.1097/QAI.0000000000002386. - DOI - PubMed
-
- Heuft M.M., Houba S.M., van den Berk G.E., Smissaert van de Haere T., van Dam A.P., Dijksman L.M., Regez R.M., Brinkman K. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS. 2014;28:999–1005. doi: 10.1097/QAD.0000000000000180. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

