Immunostimulatory Profile of Cancer Cell Death by the AdV-Lumc007-Derived Oncolytic Virus 'GoraVir' in Cultured Pancreatic Cancer Cells
- PMID: 36851497
- PMCID: PMC9959036
- DOI: 10.3390/v15020283
Immunostimulatory Profile of Cancer Cell Death by the AdV-Lumc007-Derived Oncolytic Virus 'GoraVir' in Cultured Pancreatic Cancer Cells
Abstract
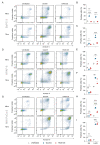
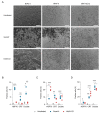
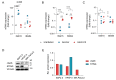
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive malignancy which shows unparalleled therapeutic resistance. Oncolytic viruses have emerged as a new treatment approach and convey their antitumor activity through lysis of cancer cells. The therapeutic efficacy of oncolytic viruses is largely dependent on the induction of immunogenic cell death (ICD) and the subsequent antitumor immune responses. However, the concurrent generation of antiviral immune responses may also limit the a virus' therapeutic window. GoraVir is a new oncolytic adenovirus derived from the Human Adenovirus B (HAdV-B) isolate AdV-lumc007 which was isolated from a gorilla and has demonstrated excellent lytic activity in both in vitro and in vivo models of PDAC. In this study, we characterized the immunostimulatory profile of cancer cell death induced by GoraVir and the concerted cellular antiviral responses in three conventional pancreatic cancer cell lines. While GoraVir was shown to induce late apoptotic/necrotic cell death at earlier time points post infection than the human adenovirus type 5 (HAdV-C5), similar levels of ICD markers were expressed. Moreover, GoraVir was shown to induce ICD not dependent on STING expression and regardless of subsequent antiviral responses. Together, these data demonstrate that GoraVir is an excellent candidate for use in oncolytic virotherapy.
Keywords: STING; immunogenic cell death; non-human primate adenovirus; oncolytic virus; pancreatic ductal adenocarcinoma.
Conflict of interest statement
R.C.H. and S.T.F.B are named inventors of patents pertaining to the use of human- and non-human primate-derived adenoviruses as viral vectors or as oncolytic agents. R.C.H. received and receives research funds from Janssen Vaccines & Prevention B.V. (Leiden, Netherlands) for projects on adenoviruses. The funders had no role in the design of the study; in the analyses, or interpretation of data; in the writing of the article; or in the decision to publish the results. The authors declare no other conflict of interests and have no other relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the article, apart from those disclosed.
Figures

Similar articles
-
Preclinical evaluation of the gorilla-derived HAdV-B AdV-lumc007 oncolytic adenovirus 'GoraVir' for the treatment of pancreatic ductal adenocarcinoma.Mol Oncol. 2024 May;18(5):1245-1258. doi: 10.1002/1878-0261.13561. Epub 2024 Jan 24. Mol Oncol. 2024. PMID: 38037840 Free PMC article.
-
Is oncolytic adenoviral-mediated immunotherapy through p53-overexpression the solution to refractory pancreatic ductal adenocarcinoma?Expert Rev Gastroenterol Hepatol. 2024 Jun;18(6):223-226. doi: 10.1080/17474124.2024.2363222. Epub 2024 Jun 17. Expert Rev Gastroenterol Hepatol. 2024. PMID: 38818792 Review.
-
Intertumoral heterogeneity impacts oncolytic vesicular stomatitis virus efficacy in mouse pancreatic cancer cells.J Virol. 2023 Sep 28;97(9):e0100523. doi: 10.1128/jvi.01005-23. Epub 2023 Sep 6. J Virol. 2023. PMID: 37671865 Free PMC article.
-
Advances in oncolytic adenovirus therapy for pancreatic cancer.Cancer Lett. 2018 Oct 10;434:56-69. doi: 10.1016/j.canlet.2018.07.006. Epub 2018 Jul 5. Cancer Lett. 2018. PMID: 29981812 Review.
-
Patient-derived pancreatic tumour organoids identify therapeutic responses to oncolytic adenoviruses.EBioMedicine. 2020 Jun;56:102786. doi: 10.1016/j.ebiom.2020.102786. Epub 2020 May 24. EBioMedicine. 2020. PMID: 32460166 Free PMC article.
Cited by
-
Biomarker screen for efficacy of oncolytic virotherapy in patient-derived pancreatic cancer cultures.EBioMedicine. 2024 Jul;105:105219. doi: 10.1016/j.ebiom.2024.105219. Epub 2024 Jun 27. EBioMedicine. 2024. PMID: 38941955 Free PMC article.
References
-
- Johns A.C., Wei L., Grogan M., Hoyd R., Bridges J.F.P., Patel S.H., Li M., Husain M., Kendra K.L., Otterson G.A., et al. Checkpoint inhibitor immunotherapy toxicity and overall survival among older adults with advanced cancer. J. Geriatr. Oncol. 2021;12:813–819. doi: 10.1016/j.jgo.2021.02.002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

