Clinical Outcomes of SARS-CoV-2 Breakthrough Infections in Liver Transplant Recipients during the Omicron Wave
- PMID: 36851510
- PMCID: PMC9958724
- DOI: 10.3390/v15020297
Clinical Outcomes of SARS-CoV-2 Breakthrough Infections in Liver Transplant Recipients during the Omicron Wave
Abstract
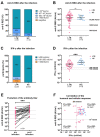
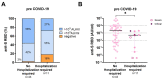
At the start of the pandemic, liver transplant recipients (LTR) were at high risk of developing severe COVID-19. Here, the outcomes of breakthrough infections in fully vaccinated LTR (n = 98) during the Omicron wave were assessed. In most patients, a mild disease course was observed, but 11 LTR (11.2%) required hospitalization for COVID-19-related complications. All patients survived. The LTR requiring hospitalization were older (67 years vs. 54 years; p < 0.001), had a higher Charlson comorbidity index (9 vs. 5; p < 0.001), and a lower anti-S RBD titer (Roche Elecsys) prior to infection (508.3 AU/mL vs. 2044 AU/mL; p = 0.03). Long-lasting symptoms for ≥4 weeks were reported by 37.5% of LTR (30/80). Risk factors in LTR included female sex (p = 0.01; Odds Ratio (OR) = 4.92 (95% confidence interval (CI) (1.5-16.5)) and dyspnea (p = 0.009; OR = 7.2 (95% CI (1.6-31.6)) during infection. Post-infection high anti-S RBD antibody levels were observed in LTR, and healthy controls (HC), while the cellular immune response, assessed by interferon-gamma release assay (EUROIMMUN), was significantly lower in LTR compared with HC (p < 0.001). In summary, in fully vaccinated LTR, SARS-CoV-2 breakthrough infections during the Omicron wave led to mild disease courses in the majority of patients and further boosted the humoral and cellular hybrid anti-SARS-CoV-2-directed immune response. While all patients survived, older and multimorbid LTR with low baseline antibody titers after vaccination still had a substantial risk for a disease course requiring hospitalization due to COVID-19-related complications.
Keywords: COVID-19; SARS-CoV-2; VOC Omicron; liver transplant recipients; post-acute COVID-19 syndrome.
Conflict of interest statement
All authors declare that they have no known competing financial, professional, or personal conflict that could have appeared to influence the work reported in this paper. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Polak W.G., Fondevila C., Karam V., Adam R., Baumann U., Germani G., Nadalin S., Taimr P., Toso C., Troisi R.I., et al. Impact of COVID-19 on liver transplantation in Europe: Alert from an early survey of European Liver and Intestine Transplantation Association and European Liver Transplant Registry. Transpl. Int. 2020;33:1244–1252. doi: 10.1111/tri.13680. - DOI - PMC - PubMed
-
- Webb G.J., Marjot T., A Cook J., Aloman C., Armstrong M.J., Brenner E.J., Catana M.-A., Cargill T., Dhanasekaran R., García-Juárez I., et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: An international registry study. Lancet Gastroenterol. Hepatol. 2020;5:1008–1016. doi: 10.1016/S2468-1253(20)30271-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

