Marburg and Ebola Virus Infections Elicit a Complex, Muted Inflammatory State in Bats
- PMID: 36851566
- PMCID: PMC9958679
- DOI: 10.3390/v15020350
Marburg and Ebola Virus Infections Elicit a Complex, Muted Inflammatory State in Bats
Abstract
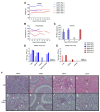
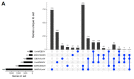
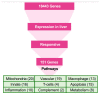
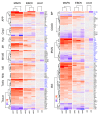
The Marburg and Ebola filoviruses cause a severe, often fatal, disease in humans and nonhuman primates but have only subclinical effects in bats, including Egyptian rousettes, which are a natural reservoir of Marburg virus. A fundamental question is why these viruses are highly pathogenic in humans but fail to cause disease in bats. To address this question, we infected one cohort of Egyptian rousette bats with Marburg virus and another cohort with Ebola virus and harvested multiple tissues for mRNA expression analysis. While virus transcripts were found primarily in the liver, principal component analysis (PCA) revealed coordinated changes across multiple tissues. Gene signatures in kidney and liver pointed at induction of vasodilation, reduction in coagulation, and changes in the regulation of iron metabolism. Signatures of immune response detected in spleen and liver indicated a robust anti-inflammatory state signified by macrophages in the M2 state and an active T cell response. The evolutionary divergence between bats and humans of many responsive genes might provide a framework for understanding the differing outcomes upon infection by filoviruses. In this study, we outline multiple interconnected pathways that respond to infection by MARV and EBOV, providing insights into the complexity of the mechanisms that enable bats to resist the disease caused by filoviral infections. The results have the potential to aid in the development of new strategies to effectively mitigate and treat the disease caused by these viruses in humans.
Keywords: Ebola virus; Marburg virus; bat; inflammation; transcriptome.
Conflict of interest statement
Author Anitha D. Jayaprakash is employed by Girihlet Inc., and author Michael F. Covington is employed by Amaryllis Nucleics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Geisbert T.W., Young H.A., Jahrling P.B., Davis K.J., Larsen T., Kagan E., Hensley L.E. Pathogenesis of Ebola hemorrhagic fever in primate models: Evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am. J. Pathol. 2003;163:2371–2382. doi: 10.1016/S0002-9440(10)63592-4. - DOI - PMC - PubMed
-
- Dhama K., Karthik K., Khandia R., Chakraborty S., Munjal A., Latheef S.K., Kumar D., Ramakrishnan M.A., Malik Y.S., Singh R., et al. Advances in Designing and Developing Vaccines, Drugs, and Therapies to Counter Ebola Virus. Front. Immunol. 2018;9:1803. doi: 10.3389/fimmu.2018.01803. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

