Establishing a New Platform to Investigate the Efficacy of Oncolytic Virotherapy in a Human Ex Vivo Peritoneal Carcinomatosis Model
- PMID: 36851574
- PMCID: PMC9963964
- DOI: 10.3390/v15020363
Establishing a New Platform to Investigate the Efficacy of Oncolytic Virotherapy in a Human Ex Vivo Peritoneal Carcinomatosis Model
Abstract
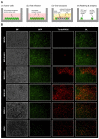
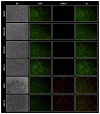
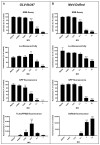
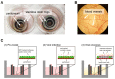
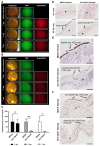
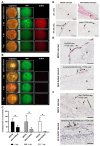
Oncolytic virotherapy constitutes a promising treatment option for many solid cancers, including peritoneal carcinomatosis (PC), which still represents a terminal stage of many types of tumors. To date, the in vitro efficacy of oncolytic viruses is mostly tested in 2D-cultured tumor cell lines due to the lack of realistic 3D in vitro tumor models. We have investigated the feasibility of virotherapy as a treatment option for PC in a human ex vivo peritoneum co-culture model. Human HT-29 cancer cells stably expressing marker genes GFP and firefly luciferase (GFP/luc) were cultured on human peritoneum and infected with two prototypic oncolytic viruses (GLV-0b347 and MeV-DsRed). Both viral constructs were able to infect HT-29 cells in patient-derived peritoneum with high tumor specificity. Over time, both GFP signal and luciferase activity decreased substantially, thereby indicating successful virus-induced oncolysis. Furthermore, immunohistochemistry stainings showed specific virotherapeutic infections of HT-29 cells and effective tumor cell lysis in infected co-cultures. Thus, the PC model established here provides a clinically relevant screening platform to evaluate the therapeutic efficacy of virotherapeutic compounds and also to investigate, in an autologous setting, the immunostimulatory potential of oncolytic viruses for PC in a unique human model system superior to standard 2D in vitro models.
Keywords: ex vivo peritoneal culture model; oncolytic measles vaccine virus; oncolytic vaccinia virus; peritoneal carcinomatosis; virotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Gene therapy using therapeutic and diagnostic recombinant oncolytic vaccinia virus GLV-1h153 for management of colorectal peritoneal carcinomatosis.Surgery. 2015 Feb;157(2):331-7. doi: 10.1016/j.surg.2014.09.008. Surgery. 2015. PMID: 25616946 Free PMC article.
-
A Three-Dimensional Organoid Model of Primary Breast Cancer to Investigate the Effects of Oncolytic Virotherapy.Front Mol Biosci. 2022 Feb 11;9:826302. doi: 10.3389/fmolb.2022.826302. eCollection 2022. Front Mol Biosci. 2022. PMID: 35223990 Free PMC article.
-
In Vivo Priming of Peritoneal Tumor-Reactive Lymphocytes With a Potent Oncolytic Virus for Adoptive Cell Therapy.Front Immunol. 2021 Feb 18;12:610042. doi: 10.3389/fimmu.2021.610042. eCollection 2021. Front Immunol. 2021. PMID: 33679747 Free PMC article.
-
Oncolytic virotherapy for oral squamous cell carcinoma using replication-competent viruses.Oral Oncol. 2009 Dec;45(12):1021-7. doi: 10.1016/j.oraloncology.2009.09.002. Epub 2009 Oct 14. Oral Oncol. 2009. PMID: 19833547 Review.
-
Measles to the Rescue: A Review of Oncolytic Measles Virus.Viruses. 2016 Oct 22;8(10):294. doi: 10.3390/v8100294. Viruses. 2016. PMID: 27782084 Free PMC article. Review.
Cited by
-
Efficacy of Different Oncolytic Vaccinia Virus Strains for the Treatment of Murine Peritoneal Mesothelioma.Cancers (Basel). 2024 Jan 15;16(2):368. doi: 10.3390/cancers16020368. Cancers (Basel). 2024. PMID: 38254857 Free PMC article.
-
Measles Virus-Based Genetic Modifications: Progress in Hematological Malignancy Treatment.Onco Targets Ther. 2025 Apr 25;18:605-615. doi: 10.2147/OTT.S518407. eCollection 2025. Onco Targets Ther. 2025. PMID: 40304006 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

