Repetitive Exposure to Bacteriophage Cocktails against Pseudomonas aeruginosa or Escherichia coli Provokes Marginal Humoral Immunity in Naïve Mice
- PMID: 36851601
- PMCID: PMC9964535
- DOI: 10.3390/v15020387
Repetitive Exposure to Bacteriophage Cocktails against Pseudomonas aeruginosa or Escherichia coli Provokes Marginal Humoral Immunity in Naïve Mice
Abstract
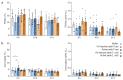
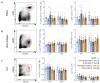
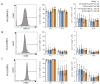
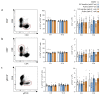
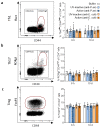
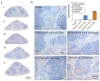
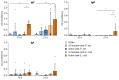
Phage therapy of ventilator-associated pneumonia (VAP) is of great interest due to the rising incidence of multidrug-resistant bacterial pathogens. However, natural or therapy-induced immunity against therapeutic phages remains a potential concern. In this study, we investigated the innate and adaptive immune responses to two different phage cocktails targeting either Pseudomonas aeruginosa or Escherichia coli-two VAP-associated pathogens-in naïve mice without the confounding effects of a bacterial infection. Active or UV-inactivated phage cocktails or buffers were injected intraperitoneally daily for 7 days in C57BL/6J wild-type mice. Blood cell analysis, flow cytometry analysis, assessment of phage distribution and histopathological analysis of spleens were performed at 6 h, 10 days and 21 days after treatment start. Phages reached the lungs and although the phage cocktails were slightly immunogenic, phage injections were well tolerated without obvious adverse effects. No signs of activation of innate or adaptive immune cells were observed; however, both active phage cocktails elicited a minimal humoral response with secretion of phage-specific antibodies. Our findings show that even repetitive injections lead only to a minimal innate and adaptive immune response in naïve mice and suggest that systemic phage treatment is thus potentially suitable for treating bacterial lung infections.
Keywords: adaptive and innate immunity; immunogenicity; phage therapy; pneumonia.
Conflict of interest statement
S.-M.W. reports non-financial support from Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin e.V., non-financial support from Mukoviszidose e.V., non-financial support from the Universität des Saarlands and personal fees from the German University in Cairo as well as personal fees and non-financial support from the Schlütersche Verlagsgesellschaft for a presentation. Unrelated to this project, G.N. received funding for research from Biotest AG and M.W. received funding for research from Bayer Health Care, Biotest AG, Pantherna, and for lectures and advisory from Actelion, Aptarion, Astra Zeneca, Bayer Health Care, Biotest AG, Boehringer Ingelheim, Chiesi, Glaxo Smith Kline, Inflarx, Insmed, Novartis, Pantherna, Pherecydes, Sinoxa and Teva. The supporting sources had no involvement in study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
Figures

References
-
- Anyaegbunam N.J., Anekpo C.C., Anyaegbunam Z.K.G., Doowuese Y., Chinaka C.B., Odo O.J., Sharndama H.C., Okeke O.P., Mba I.E. The resurgence of phage-based therapy in the era of increasing antibiotic resistance: From research progress to challenges and prospects. Microbiol. Res. 2022;264:127155. doi: 10.1016/j.micres.2022.127155. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

