Hepatitis B Blood Donor Screening Data: An Under-Recognized Resource for Canadian Public Health Surveillance
- PMID: 36851623
- PMCID: PMC9966614
- DOI: 10.3390/v15020409
Hepatitis B Blood Donor Screening Data: An Under-Recognized Resource for Canadian Public Health Surveillance
Abstract
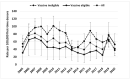
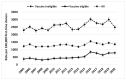
Hepatitis B surveillance is essential to achieving Canada's goal of eliminating hepatitis B by 2030. Hepatitis B rates, association of infection with vaccine age-eligibility, and risk factors were analyzed among 1,401,603 first-time Canadian blood donors from 2005 to 2020. Donors were classified as having likely chronic or likely resolved/occult infections based on hepatitis B surface antigen, anti-hepatitis B core antigen, and hepatitis B nucleic acid test results. Likely chronically infected and control donors (ratio 1:4) participated in risk-factor interviews. The 2019 rate of likely chronic infection was 61.9 per 100,000 (95% CI 46.5-80.86) and 1449.5 per 100,000 for likely resolved/occult infections (95% CI 1370.7-1531.7). Likely chronic infections were higher in males (OR 3.2; 95% CI 2.7-3.7) and the vaccine-ineligible birth cohort (OR 1.9; 95% CI 1.6-2.2). The main risk factors were living with someone who had hepatitis (OR 12.5; 95% CI 5.2-30.0) and ethnic origin from a high-prevalence country (OR 8.4; 95% CI 5.9-11.9). Undiagnosed chronic hepatitis B may be more prevalent in Canada than currently determined by traditional passive hepatitis B reporting. Blood donor data can be useful in informing hepatitis B rates and evaluating vaccination programs in Canada.
Keywords: blood donors; chronic hepatitis B; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Epidemiology of hepatitis B in Canadian blood donors.Transfusion. 2008 Nov;48(11):2323-30. doi: 10.1111/j.1537-2995.2008.01845.x. Epub 2008 Jul 11. Transfusion. 2008. PMID: 18647366
-
Blood donor screening in the Netherlands: Universal anti-HBc screening in combination with HBV nucleic acid amplification testing may allow discontinuation of hepatitis B virus antigen testing.Transfusion. 2021 Jul;61(7):2116-2124. doi: 10.1111/trf.16420. Epub 2021 Apr 26. Transfusion. 2021. PMID: 33899233
-
An assessment of hepatitis B virus prevalence in South African young blood donors born after the implementation of the infant hepatitis B virus immunization program: Implications for transfusion safety.Transfusion. 2021 Sep;61(9):2688-2700. doi: 10.1111/trf.16559. Epub 2021 Jun 26. Transfusion. 2021. PMID: 34173987 Free PMC article.
-
New strategies for blood donor screening for hepatitis B virus: nucleic acid testing versus immunoassay methods.Mol Diagn Ther. 2006;10(2):77-91. doi: 10.1007/BF03256447. Mol Diagn Ther. 2006. PMID: 16669606 Review.
-
Occult hepatitis B virus infection.Transfus Clin Biol. 2004 Feb;11(1):18-25. doi: 10.1016/j.tracli.2003.11.007. Transfus Clin Biol. 2004. PMID: 14980545 Review.
Cited by
-
Influence of Occult Hepatitis B Infection on Blood Transfusion Safety and Its Countermeasures.Pathogens. 2025 Mar 21;14(4):301. doi: 10.3390/pathogens14040301. Pathogens. 2025. PMID: 40333041 Free PMC article. Review.
-
SARS-CoV-2 vaccination in Canadian blood donors: Insight into donor representativeness of the general population.Vaccine X. 2024 May 12;18:100498. doi: 10.1016/j.jvacx.2024.100498. eCollection 2024 Jun. Vaccine X. 2024. PMID: 38800670 Free PMC article.
-
Notification of blood donors who test positive for transfusion-transmissible infections.Vox Sang. 2025 Apr;120(4):394-400. doi: 10.1111/vox.13796. Epub 2025 Jan 14. Vox Sang. 2025. PMID: 39809317 Free PMC article.
-
Influenza and COVID-19 vaccination in Canadian blood donors: A comparison across pre- and post-pandemic periods.Vox Sang. 2025 May;120(5):464-472. doi: 10.1111/vox.70006. Epub 2025 Feb 27. Vox Sang. 2025. PMID: 40015315 Free PMC article.
-
Serological and Molecular Characterization of Occult HBV Infection in Blood Donors from South Italy.Viruses. 2023 Dec 31;16(1):71. doi: 10.3390/v16010071. Viruses. 2023. PMID: 38257771 Free PMC article.
References
-
- National Advisory Committee on Immunization (NACI) Update on the Recommended Use of Hepatitis B Vaccine. [(accessed on 19 December 2022)]; Available online: https://www.canada.ca/content/dam/phac-aspc/documents/services/publicati....
-
- Wong P.H.P. Chronic hepatitis B in British Columbia. Br. Columbia Med. J. 2022;64:2130217.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

