Stratification of COVID-19 Severity Using SeptiCyte RAPID, a Novel Host Immune Response Test
- PMID: 36851633
- PMCID: PMC9960895
- DOI: 10.3390/v15020419
Stratification of COVID-19 Severity Using SeptiCyte RAPID, a Novel Host Immune Response Test
Abstract
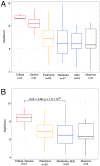
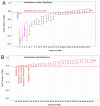
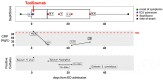
SeptiCyte® RAPID is a gene expression assay measuring the relative expression levels of host response genes PLA2G7 and PLAC8, indicative of a dysregulated immune response during sepsis. As severe forms of COVID-19 may be considered viral sepsis, we evaluated SeptiCyte RAPID in a series of 94 patients admitted to Foch Hospital (Suresnes, France) with proven SARS-CoV-2 infection. EDTA blood was collected in the emergency department (ED) in 67 cases, in the intensive care unit (ICU) in 23 cases and in conventional units in 4 cases. SeptiScore (0-15 scale) increased with COVID-19 severity. Patients in ICU had the highest SeptiScores, producing values comparable to 8 patients with culture-confirmed bacterial sepsis. Receiver operating characteristic (ROC) curve analysis had an area under the curve (AUC) of 0.81 for discriminating patients requiring ICU admission from patients who were immediately discharged or from patients requiring hospitalization in conventional units. SeptiScores increased with the extent of the lung injury. For 68 patients, a chest computed tomography (CT) scan was performed within 24 h of COVID-19 diagnosis. SeptiScore >7 suggested lung injury ≥50% (AUC = 0.86). SeptiCyte RAPID was compared to other biomarkers for discriminating Critical + Severe COVID-19 in ICU, versus Moderate + Mild COVID-19 not in ICU. The mean AUC for SeptiCyte RAPID was superior to that of any individual biomarker or combination thereof. In contrast to C-reactive protein (CRP), correlation of SeptiScore with lung injury was not impacted by treatment with anti-inflammatory agents. SeptiCyte RAPID can be a useful tool to identify patients with severe forms of COVID-19 in ED, as well as during follow-up.
Keywords: COVID-19; gene expression; severity; viral sepsis.
Conflict of interest statement
T.D.Y., K.N. and J.T.K. are current or former employees and shareholders in Immunexpress. The other authors declare no competing interests. The DRIVe Solving Sepsis program of the Biomedical Advanced Research and Development Authority (BARDA) had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Shappell C.N., Klompas M.M., Kanjilal S.M., Chan C.M., Rhee C.M. Prevalence, Clinical Characteristics, and Outcomes of Sepsis Caused by Severe Acute Respiratory Syndrome Coronavirus 2 Versus Other Pathogens in Hospitalized Patients with COVID-19. Crit. Care Explor. 2022;4:e0703. doi: 10.1097/CCE.0000000000000703. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

