Pseudomonas Phage ZCPS1 Endolysin as a Potential Therapeutic Agent
- PMID: 36851734
- PMCID: PMC9961711
- DOI: 10.3390/v15020520
Pseudomonas Phage ZCPS1 Endolysin as a Potential Therapeutic Agent
Abstract
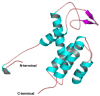
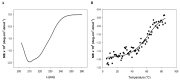
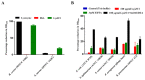
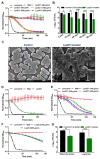
The challenge of antibiotic resistance has gained much attention in recent years due to the rapid emergence of resistant bacteria infecting humans and risking industries. Thus, alternatives to antibiotics are being actively searched for. In this regard, bacteriophages and their enzymes, such as endolysins, are a very attractive alternative. Endolysins are the lytic enzymes, which are produced during the late phase of the lytic bacteriophage replication cycle to target the bacterial cell walls for progeny release. Here, we cloned, expressed, and purified LysZC1 endolysin from Pseudomonas phage ZCPS1. The structural alignment, molecular dynamic simulation, and CD studies suggested LysZC1 to be majorly helical, which is highly similar to various phage-encoded lysozymes with glycoside hydrolase activity. Our endpoint turbidity reduction assay displayed the lytic activity against various Gram-positive and Gram-negative pathogens. Although in synergism with EDTA, LysZC1 demonstrated significant activity against Gram-negative pathogens, it demonstrated the highest activity against Bacillus cereus. Moreover, LysZC1 was able to reduce the numbers of logarithmic-phase B. cereus by more than 2 log10 CFU/mL in 1 h and also acted on the stationary-phase culture. Remarkably, LysZC1 presented exceptional thermal stability, pH tolerance, and storage conditions, as it maintained the antibacterial activity against its host after nearly one year of storage at 4 °C and after being heated at temperatures as high as 100 °C for 10 min. Our data suggest that LysZC1 is a potential candidate as a therapeutic agent against bacterial infection and an antibacterial bio-control tool in food preservation technology.
Keywords: antibacterial tool; antibiotic resistance; bacteriophages; foodborne pathogens; phage-encoded enzymes; thermo-stable.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

