Therapeutic treatment with fluoxetine using the chronic unpredictable stress model induces changes in neurotransmitters and circulating miRNAs in extracellular vesicles
- PMID: 36852042
- PMCID: PMC9958461
- DOI: 10.1016/j.heliyon.2023.e13442
Therapeutic treatment with fluoxetine using the chronic unpredictable stress model induces changes in neurotransmitters and circulating miRNAs in extracellular vesicles
Abstract
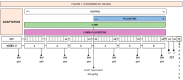
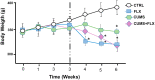
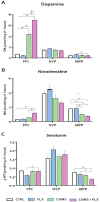
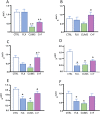
The most widely prescribed antidepressant, fluoxetine (FLX), is known for its antioxidant and anti-inflammatory effects when administered post-stress. Few studies have evaluated the effects of FLX treatment when chronic stress has induced deleterious effects in patients. Our objective was to evaluate FLX treatment (20 mg/kg/day, i.v.) once these effects are manifested, and the drug's relation to extracellular circulating microRNAs associated with inflammation, a hedonic response (sucrose intake), the forced swim test (FST), and corticosterone levels (CORT) and monoamine concentrations in limbic areas. A group of Wistar rats was divided into groups: Control; FLX; CUMS (for six weeks of exposure to chronic, unpredictable mild stress); and CUMS + FLX, a mixed group. After CUMS, the rats performed the FST, and serum levels of CORT and six microRNAs (miR-16, -21, -144, -155, -146a, -223) were analyzed, as were levels of dopamine, noradrenaline, and serotonin in the prefrontal cortex, hippocampus, and hypothalamus. CUMS reduced body weight, sucrose intake, and hippocampal noradrenaline levels, but increased CORT, immobility behavior on the FST, dopamine concentrations in the prefrontal cortex, and all miRNAs except miR-146a expression. Administering FLX during CUMS reduced CORT levels and immobility behavior on the FST and increased the expression of miR-16, -21, -146a, -223, and dopamine. FLX protects against the deleterious effects of stress by reducing CORT and has an antidepressant effect on the FST, with minimally-modified neurotransmitter levels. FLX increased the expression of miRNAs as part of the antidepressant effect. It also regulates both neuroinflammation and serotoninergic neurotransmission through miRNAs, such as the miR-16.
Keywords: Chronic unpredictable mild stress; Corticosterone; Fluoxetine; Neurotransmitters; Serotonin; miRNAs.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
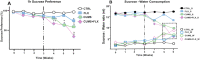

Similar articles
-
Parity modifies the effects of fluoxetine and corticosterone on behavior, stress reactivity, and hippocampal neurogenesis.Neuropharmacology. 2016 Jun;105:443-453. doi: 10.1016/j.neuropharm.2015.11.027. Epub 2016 Jan 22. Neuropharmacology. 2016. PMID: 26808316
-
18β-Glycyrrhetinic Acid Ameliorates Neuroinflammation Linked Depressive Behavior Instigated by Chronic Unpredictable Mild Stress via Triggering BDNF/TrkB Signaling Pathway in Rats.Neurochem Res. 2023 Feb;48(2):551-569. doi: 10.1007/s11064-022-03779-7. Epub 2022 Oct 28. Neurochem Res. 2023. PMID: 36307572 Free PMC article.
-
Antidepressant-Like Effect of Lipid Extract of Channa striatus in Chronic Unpredictable Mild Stress Model of Depression in Rats.Evid Based Complement Alternat Med. 2016;2016:2986090. doi: 10.1155/2016/2986090. Epub 2016 Dec 18. Evid Based Complement Alternat Med. 2016. PMID: 28074100 Free PMC article.
-
Omega-3 fatty acid deficiency does not alter the effects of chronic fluoxetine treatment on central serotonin turnover or behavior in the forced swim test in female rats.Pharmacol Biochem Behav. 2013 Dec;114-115:1-8. doi: 10.1016/j.pbb.2013.09.010. Epub 2013 Oct 1. Pharmacol Biochem Behav. 2013. PMID: 24090922 Free PMC article.
-
Antidepressant-like activity of hyperforin and changes in BDNF and zinc levels in mice exposed to chronic unpredictable mild stress.Behav Brain Res. 2019 Oct 17;372:112045. doi: 10.1016/j.bbr.2019.112045. Epub 2019 Jun 17. Behav Brain Res. 2019. PMID: 31220487
Cited by
-
MiR-9-enriched mesenchymal stem cells derived exosomes prevent cystitis-induced bladder pain via suppressing TLR4/NLRP3 pathway in interstitial cystitis mice.Immun Inflamm Dis. 2024 Feb;12(2):e1140. doi: 10.1002/iid3.1140. Immun Inflamm Dis. 2024. PMID: 38415918 Free PMC article.
-
Non-Categorical Analyses Identify Rotenone-Induced 'Parkinsonian' Rats Benefiting from Nano-Emulsified Punicic Acid (Nano-PSO) in a Phenotypically Diverse Population: Implications for Translational Neurodegenerative Therapies.Int J Mol Sci. 2024 Nov 25;25(23):12635. doi: 10.3390/ijms252312635. Int J Mol Sci. 2024. PMID: 39684350 Free PMC article.
References
-
- Korte S.M., Prins J., Krajnc A.M., Hendriksen H., Oosting R.S., Westphal K.G., Korte-Bouws G.A., Olivier B. The many different faces of major depression: it is time for personalized medicine. Eur. J. Pharmacol. 2015 Dec;753:88–104. - PubMed
-
- WHO . 2020. Depression [WWW document]https://www.who.int/news-room/fact-sheets/detail/depression
-
- Romanczuk-Seiferth N., Pöhland L., Mohnke S., Garbusow M., Erk S., Haddad L., Grimm O., Tost H., Meyer-Lindenberg A., Walter H., Wüstenberg T., Heinz A. Larger amygdala volume in first-degree relatives of patients with major depression. NeuroImage Clin. 2014 Jun;5:62–68. doi: 10.1016/j.nicl.2014.05.015. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

