The prognostic value and immunological role of SULF2 in adrenocortical carcinoma
- PMID: 36852051
- PMCID: PMC9957709
- DOI: 10.1016/j.heliyon.2023.e13613
The prognostic value and immunological role of SULF2 in adrenocortical carcinoma
Abstract
Background: Adrenocortical carcinoma (ACC) represents the rare urological epithelial cancer of urinary tract, which has a large mass and is usually diagnosed at the advanced stage, thus inducing the poor prognosis. As a result, early detection and diagnosis are more important for the prognosis rather than the treatment of ACC. There is evidence supporting the association of Sulfatase2 (SULF2) with bladder cancer. However, the relationships of SULF2 with the clinical features and immune infiltration of ACC remain unclear.
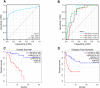
Methods: This work comprehensively investigated the different expression levels of SULF2 within ACC and its prognostic significance through various databases including Gene Expression Profiling Interaction Analysis (GEPIA), Tumor Immune Estimation Resource (TIMER), The Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO), Kaplan-Meier (KM) plotter and UALCAN. Besides, SULF2 levels within different tumor and paraneoplastic tissues were examined based on Human Protein Atlas (HPA) and TIMER. Afterwards, this study identified differentially expressed genes (DEGs) in high-compared with low-SULF2-expression groups. To predict the possible interaction between SULF2 and its targets, a protein-protein interaction (PPI) network was constructed based on relevant data collected in STRING database. Besides, the SULF2 functional annotation was carried out, including Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) and GSEA. In addition, gene mutation analysis was also performed based on the cBioPortal database. The relation of SULF2 with immune infiltration was analyzed from various aspects by using the resources of various databases including TIMER, TISIDB, and GEPIA, which was first reported in this work. Finally, R package was utilized to plot the receiver operating characteristic (ROC) curves of diagnosis, time-dependent survival, and the association of SULF2 with cancer stage and the nomogram model. Finally, CellMiner dataset was adopted for SULF2 correlation as well as drug sensitivity analysis.
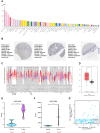
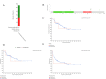
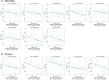
Results: Relative to healthy people, SULF2 level markedly elevated within ACC tissues. Besides, SULF2 up-regulation significantly predicted the dismal prognostic outcome, which may be an important prognostic factor. Afterwards, the PPI network was constructed, and the possible link of SULF2 with the corresponding targets was predicted. Besides, up-regulated SULF2 expression was tightly related to immune regulation and tumor-infiltration immune cell (TIICs), including CD8+, CD4+ and mast cells. Finally, SULF2 expression was speculated to help determine the sensitivity of certain drugs.
Conclusions: SULF2 may offer a new therapeutic target for ACC patients and become an important potential prognostic biomarker.
Keywords: Adrenocortical carcinoma (ACC); Prognosis; Sulfatase2 (SULF2); Tumor immune regulation.
© 2023 The Authors.
Conflict of interest statement
All authors declared no competing interest.
Figures







Similar articles
-
High expression of PDZ-binding kinase is correlated with poor prognosis and immune infiltrates in hepatocellular carcinoma.World J Surg Oncol. 2022 Jan 22;20(1):22. doi: 10.1186/s12957-021-02479-w. World J Surg Oncol. 2022. PMID: 35065633 Free PMC article.
-
Identification of SHCBP1 as a potential biomarker involving diagnosis, prognosis, and tumor immune microenvironment across multiple cancers.Comput Struct Biotechnol J. 2022 Jun 18;20:3106-3119. doi: 10.1016/j.csbj.2022.06.039. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35782736 Free PMC article.
-
Prediction and analysis of novel key genes ITGAX, LAPTM5, SERPINE1 in clear cell renal cell carcinoma through bioinformatics analysis.PeerJ. 2021 Apr 20;9:e11272. doi: 10.7717/peerj.11272. eCollection 2021. PeerJ. 2021. PMID: 33976979 Free PMC article.
-
Golgi scaffold protein PAQR11 in pan-cancer landscape: A comprehensive bioinformatics exploration of expression patterns, prognostic significance, and potential immunological function.Heliyon. 2025 Jan 4;11(2):e41724. doi: 10.1016/j.heliyon.2025.e41724. eCollection 2025 Jan 30. Heliyon. 2025. PMID: 39906812 Free PMC article. Review.
-
Current Issues in the Diagnosis and Management of Adrenocortical Carcinomas.2021 Aug 30. In: Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. 2021 Aug 30. In: Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. PMID: 25905240 Free Books & Documents. Review.
References
-
- Fassnacht M., et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31(11):1476–1490. - PubMed
-
- Zhang Z., et al. Expression and clinical significance of VISTA and PD-L1 in adrenocortical carcinoma. Endocr. Relat. Cancer. 2022;29(7):403–413. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

