Fast and efficient identification of hyaluronidase specific inhibitors from Chrysanthemum morifolium Ramat. using UF-LC-MS technique and their anti-inflammation effect in macrophages
- PMID: 36852058
- PMCID: PMC9957760
- DOI: 10.1016/j.heliyon.2023.e13709
Fast and efficient identification of hyaluronidase specific inhibitors from Chrysanthemum morifolium Ramat. using UF-LC-MS technique and their anti-inflammation effect in macrophages
Abstract
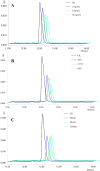
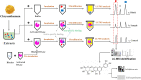
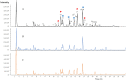
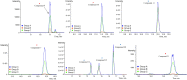
The purpose of the study was to establish a rapid analytical strategy to screen potential anti-inflammatory compounds from Flos Chrysanthemum flower. The enzyme assay was conducted to prescreen botanical extracts, in which Chrysanthemum morifolium aqueous extract (CME) displayed hyaluronidase (HAase) inhibitory activity in a dose-dependent manner with the values of 8.31, 24.25, and 66.51% at concentrations of 1.00, 2.00, and 4 0.00 mg/mL, respectively. Eight potential compounds targeting HAase (compounds 9, 10, 11, 13, 15, 17, 20 and 21) from CME were screened using ultrafiltration affinity liquid chromatography coupled with mass spectrometry (UF-LC-MS) technology. The well-known inhibitor, dipotassium glycyrrhizinate (DG), was used as a positive control and competitive ligand to eliminate false positives. Then, four of these potential components (compounds 9, 10, 17, and 21), namely eriodictyol-7-O-glucoside, luteoloside, apigenin-7-O-glucoside and diosmetin-7-O-glucoside, were distinguished as potent HAase specific inhibitor candidates with high BD and CBD values. The enzyme inhibitory activities of candidate compounds were verified using enzyme inhibition assay. At a concentration of 1000 μM, compounds 9, 10, 17, and 21 showed 40.15, 44.85, 18.04, and 24.15% inhibition of HAase, respectively. Furthermore, all the four compounds significantly decreased the production of nitric oxide (NO) and IL-6, and significantly suppressed the mRNA expression of inducible NO synthase (iNOS) and IL-1β in both murine and human macrophages.
Keywords: Anti-inflammation; Chrysanthemum morifolium; Hyaluronidase inhibitors; Macrophages; UF-UPLC-Q-TOF-MS.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Screening for selective inhibitors of xanthine oxidase from Flos Chrysanthemum using ultrafiltration LC-MS combined with enzyme channel blocking.J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Jun 15;961:56-61. doi: 10.1016/j.jchromb.2014.05.001. Epub 2014 May 14. J Chromatogr B Analyt Technol Biomed Life Sci. 2014. PMID: 24861872
-
Targeted screening of multiple anti-inflammatory components from Chrysanthemi indici Flos by ligand fishing with affinity UF-LC/MS.Front Pharmacol. 2024 Apr 16;15:1272087. doi: 10.3389/fphar.2024.1272087. eCollection 2024. Front Pharmacol. 2024. PMID: 38694923 Free PMC article.
-
Insights into the importance of dietary chrysanthemum flower (Chrysanthemum morifolium cv. Hangju)-wolfberry (Lycium barbarum fruit) combination in antioxidant and anti-inflammatory properties.Food Res Int. 2019 Feb;116:810-818. doi: 10.1016/j.foodres.2018.09.015. Epub 2018 Sep 10. Food Res Int. 2019. PMID: 30717012
-
Screening of key flavonoids and monoterpenoids for xanthine oxidase inhibitory activity-oriented quality control of Chrysanthemum morifolium Ramat. 'Boju' based on spectrum-effect relationship coupled with UPLC-TOF-MS and HS-SPME-GC/MS.Food Res Int. 2020 Nov;137:109448. doi: 10.1016/j.foodres.2020.109448. Epub 2020 Jun 17. Food Res Int. 2020. PMID: 33233127
-
The flower head of Chrysanthemum morifolium Ramat. (Juhua): A paradigm of flowers serving as Chinese dietary herbal medicine.J Ethnopharmacol. 2020 Oct 28;261:113043. doi: 10.1016/j.jep.2020.113043. Epub 2020 Jun 25. J Ethnopharmacol. 2020. PMID: 32593689 Review.
Cited by
-
Elderberry Leaves with Antioxidant and Anti-Inflammatory Properties as a Valuable Plant Material for Wound Healing.Pharmaceuticals (Basel). 2024 May 10;17(5):618. doi: 10.3390/ph17050618. Pharmaceuticals (Basel). 2024. PMID: 38794188 Free PMC article.
-
Chrysanthemum morifolium Ramat extract and probiotics combination ameliorates metabolic disorders through regulating gut microbiota and PPARα subcellular localization.Chin Med. 2024 Jun 3;19(1):76. doi: 10.1186/s13020-024-00950-w. Chin Med. 2024. PMID: 38831430 Free PMC article.
-
Exploring the antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory properties of Chrysanthemum morifolium and Chrysanthemum indicum: a narrow review.Front Pharmacol. 2025 Mar 19;16:1538311. doi: 10.3389/fphar.2025.1538311. eCollection 2025. Front Pharmacol. 2025. PMID: 40176916 Free PMC article. Review.
-
Hydrogen Protection Boosts the Bioactivity of Chrysanthemum morifolium Extract in Preventing Palmitate-Induced Endothelial Dysfunction by Restoring MFN2 and Alleviating Oxidative Stress in HAEC Cells.Antioxidants (Basel). 2023 Apr 28;12(5):1019. doi: 10.3390/antiox12051019. Antioxidants (Basel). 2023. PMID: 37237885 Free PMC article.
-
Distinct chemical structures inhibit the CEMIP hyaluronidase and promote oligodendrocyte progenitor cell maturation.J Biol Chem. 2024 Dec;300(12):107916. doi: 10.1016/j.jbc.2024.107916. Epub 2024 Oct 24. J Biol Chem. 2024. PMID: 39454959 Free PMC article.
References
-
- Peng A., et al. Classification of edible chrysanthemums based on phenolic profiles and mechanisms underlying the protective effects of characteristic phenolics on oxidatively damaged erythrocyte. Food Res. Int. 2019;123:64–74. - PubMed
-
- Lin L.-Z., Harnly J.M. Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat) Food Chem. 2010;120(1):319–326.
-
- Zhang Z., et al. On-line screening of natural antioxidants and the antioxidant activity prediction for the extracts from flowers of Chrysanthemum morifolium ramat. J. Ethnopharmacol. 2022;294 - PubMed
-
- Zhang N., et al. Insights into the importance of dietary chrysanthemum flower (Chrysanthemum morifolium cv. Hangju)-wolfberry (Lycium barbarum fruit) combination in antioxidant and anti-inflammatory properties. Food Res. Int. 2019;116:810–818. - PubMed
-
- Wang S., et al. Study on the effects of sulfur fumigation on chemical constituents and antioxidant activity of Chrysanthemum morifolium cv. Hang-ju. Phytomedicine. 2014;21(5):773–779. - PubMed
LinkOut - more resources
Full Text Sources

