Efficient control of the fungal pathogens Colletotrichum gloeosporioides and Penicillium digitatum infecting citrus fruits by native soilborne Bacillus velezensis strains
- PMID: 36852059
- PMCID: PMC9958435
- DOI: 10.1016/j.heliyon.2023.e13663
Efficient control of the fungal pathogens Colletotrichum gloeosporioides and Penicillium digitatum infecting citrus fruits by native soilborne Bacillus velezensis strains
Abstract
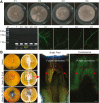
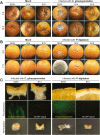
Destruction of citrus fruits by fungal pathogens during preharvest and postharvest stages can result in severe losses for the citrus industry. Antagonistic microorganisms used as biological agents to control citrus pathogens are considered alternatives to synthetic fungicides. In this study, we aimed to identify fungal pathogens causing dominant diseases on citrus fruits in a specialized citrus cultivation region of Vietnam and inspect soilborne Bacillus isolates with antifungal activity against these pathogens. Two fungal pathogens were characterized as Colletotrichum gloeosporioides and Penicillium digitatum based on morphological characteristics and ribosomal DNA internal transcribed spacer sequence analyses. Reinfection assays of orange fruits confirmed that C. gloeosporioides causes stem-end rot, and P. digitatum triggers green mold disease. By the heterologous expression of the green fluorescent protein (GFP) in C. gloeosporioides using Agrobacterium tumefaciens-mediated transformation, we could observe the fungal infection process of the citrus fruit stem-end rot caused by C. gloeosporioides for the first time. Furthermore, we isolated and selected two soilborne Bacillus strains with strong antagonistic activity for preventing the decay of citrus fruits by these pathogens. Molecular analyses of 16 S rRNA and gyrB genes showed that both isolates belong to B. velezensis. Antifungal activity assays indicated that bacterial culture suspensions could strongly inhibit C. gloeosporioides and P. digitatum, and shield orange fruits from the invasion of the pathogens. Our work provides a highly effective Bacillus-based preservative solution for combating the fungal pathogens C. gloeosporioides and P. digitatum to protect citrus fruits at the postharvest stages.
Keywords: Antifungal activity; Bacillus velezensis; Citrus fruits; Colletotrichum gloeosporioides; GFP tagging; Host colonization; Penicillium digitatum; Postharvest pathogens.
© 2023 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A highly efficient Agrobacterium tumefaciens-mediated transformation system for the postharvest pathogen Penicillium digitatum using DsRed and GFP to visualize citrus host colonization.J Microbiol Methods. 2018 Jan;144:134-144. doi: 10.1016/j.mimet.2017.11.019. Epub 2017 Nov 23. J Microbiol Methods. 2018. PMID: 29175534
-
Effectiveness of Trichoderma strains isolated from the rhizosphere of citrus tree to control Alternaria alternata, Colletotrichum gloeosporioides and Penicillium digitatum A21 resistant to pyrimethanil in post-harvest oranges (Citrus sinensis L. (Osbeck)).J Appl Microbiol. 2020 Sep;129(3):712-727. doi: 10.1111/jam.14657. Epub 2020 Apr 29. J Appl Microbiol. 2020. PMID: 32249987
-
Phenylalanine: A Promising Inducer of Fruit Resistance to Postharvest Pathogens.Foods. 2020 May 18;9(5):646. doi: 10.3390/foods9050646. Foods. 2020. PMID: 32443417 Free PMC article.
-
Penicillium digitatum infection mechanisms in citrus: What do we know so far?Fungal Biol. 2019 Aug;123(8):584-593. doi: 10.1016/j.funbio.2019.05.004. Epub 2019 May 13. Fungal Biol. 2019. PMID: 31345412 Review.
-
Inhibition of Key Citrus Postharvest Fungal Strains by Plant Extracts In Vitro and In Vivo: A Review.Plants (Basel). 2019 Jan 22;8(2):26. doi: 10.3390/plants8020026. Plants (Basel). 2019. PMID: 30678206 Free PMC article. Review.
Cited by
-
Ethanolic extract from fruiting bodies of Cordyceps militaris HL8 exhibits cytotoxic activities against cancer cells, skin pathogenic yeasts, and postharvest pathogen Penicillium digitatum.Arch Microbiol. 2024 Feb 13;206(3):97. doi: 10.1007/s00203-024-03833-8. Arch Microbiol. 2024. PMID: 38349544
-
Comparative study of antioxidant and antimicrobial activity of berberine-derived Schiff bases, nitro-berberine and amino-berberine.Heliyon. 2023 Nov 25;9(12):e22783. doi: 10.1016/j.heliyon.2023.e22783. eCollection 2023 Dec. Heliyon. 2023. PMID: 38058428 Free PMC article.
-
Morphological, Physiological, Biochemical, and Molecular Characterization of Fungal Species Associated with Papaya Rot in Cameroon.J Fungi (Basel). 2025 May 17;11(5):385. doi: 10.3390/jof11050385. J Fungi (Basel). 2025. PMID: 40422720 Free PMC article.
-
Biocontrol of citrus fungal pathogens by lipopeptides produced by Bacillus velezensis TZ01.Front Microbiol. 2024 Sep 4;15:1471305. doi: 10.3389/fmicb.2024.1471305. eCollection 2024. Front Microbiol. 2024. PMID: 39296284 Free PMC article.
-
Combined transcriptomic and metabolomic analysis of the mechanism by which Bacillus velezensis induces resistance to anthracnose in walnut.Front Microbiol. 2024 Oct 9;15:1420922. doi: 10.3389/fmicb.2024.1420922. eCollection 2024. Front Microbiol. 2024. PMID: 39444687 Free PMC article.
References
-
- FAO . Rome; 2021. Citrus Fruit Statistical Compendium 2020.http://www.fao.org/3/cb6492en/cb6492en.pdf
-
- Dowling M., Peres N., Villani S., Schnabel G. Managing Colletotrichum on fruit crops: a “complex” challenge. Plant Dis. 2020;104:2301–2316. - PubMed
-
- Lima W.G., Spósito M.B., Amorim L., Gonçalves F.P., de Filho P.A.M. Colletotrichum gloeosporioides, a new causal agent of citrus post-bloom fruit drop. Eur. J. Plant Pathol. 2011;131:157–165.
-
- Zakaria L. Diversity of Colletotrichum species associated with anthracnose disease in tropical fruit crops − A review. Agriculture. 2021;11:297.
LinkOut - more resources
Full Text Sources

