Forced enhancer-promoter rewiring to alter gene expression in animal models
- PMID: 36852088
- PMCID: PMC9958407
- DOI: 10.1016/j.omtn.2023.01.016
Forced enhancer-promoter rewiring to alter gene expression in animal models
Abstract
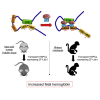
Transcriptional enhancers can be in physical proximity of their target genes via chromatin looping. The enhancer at the β-globin locus (locus control region [LCR]) contacts the fetal-type (HBG) and adult-type (HBB) β-globin genes during corresponding developmental stages. We have demonstrated previously that forcing proximity between the LCR and HBG genes in cultured adult-stage erythroid cells can activate HBG transcription. Activation of HBG expression in erythroid cells is of benefit to patients with sickle cell disease. Here, using the β-globin locus as a model, we provide proof of concept at the organismal level that forced enhancer rewiring might present a strategy to alter gene expression for therapeutic purposes. Hematopoietic stem and progenitor cells (HSPCs) from mice bearing human β-globin genes were transduced with lentiviral vectors expressing a synthetic transcription factor (ZF-Ldb1) that fosters LCR-HBG contacts. When engrafted into host animals, HSPCs gave rise to adult-type erythroid cells with elevated HBG expression. Vectors containing ZF-Ldb1 were optimized for activity in cultured human and rhesus macaque erythroid cells. Upon transplantation into rhesus macaques, erythroid cells from HSPCs expressing ZF-Ldb1 displayed elevated HBG production. These findings in two animal models suggest that forced redirection of gene-regulatory elements may be used to alter gene expression to treat disease.
Keywords: MT: Oligonucleotides; Therapies and Applications; enhancer-promoter interactions; fetal hemoglobin; forced chromatin looping; hemoglobin switching; preclinical animal models; sickle cell disease; vector optimization.
© 2023 The Author(s).
Conflict of interest statement
G.A.B. has received research funding from Bioverativ and Pfizer, Inc.
Figures




Similar articles
-
Forced chromatin looping raises fetal hemoglobin in adult sickle cells to higher levels than pharmacologic inducers.Blood. 2016 Aug 25;128(8):1139-43. doi: 10.1182/blood-2016-01-691089. Epub 2016 Jul 12. Blood. 2016. PMID: 27405777 Free PMC article.
-
LDB1-mediated enhancer looping can be established independent of mediator and cohesin.Nucleic Acids Res. 2017 Aug 21;45(14):8255-8268. doi: 10.1093/nar/gkx433. Nucleic Acids Res. 2017. PMID: 28520978 Free PMC article.
-
SIRT1 activates the expression of fetal hemoglobin genes.Am J Hematol. 2017 Nov;92(11):1177-1186. doi: 10.1002/ajh.24879. Epub 2017 Aug 28. Am J Hematol. 2017. PMID: 28776729 Free PMC article.
-
Reactivation of developmentally silenced globin genes by forced chromatin looping.Cell. 2014 Aug 14;158(4):849-860. doi: 10.1016/j.cell.2014.05.050. Cell. 2014. PMID: 25126789 Free PMC article.
-
Identification of a Novel Enhancer/Chromatin Opening Element Associated with High-Level γ-Globin Gene Expression.Mol Cell Biol. 2018 Sep 14;38(19):e00197-18. doi: 10.1128/MCB.00197-18. Print 2018 Oct 1. Mol Cell Biol. 2018. PMID: 30012865 Free PMC article.
Cited by
-
The cells are all-right: Regulation of the Lefty genes by separate enhancers in mouse embryonic stem cells.PLoS Genet. 2024 Dec 13;20(12):e1011513. doi: 10.1371/journal.pgen.1011513. eCollection 2024 Dec. PLoS Genet. 2024. PMID: 39671433 Free PMC article.
-
Single-stranded DNA-binding proteins are essential components of the architectural LDB1 protein complex.Mol Cell. 2025 Aug 21;85(16):3074-3089.e8. doi: 10.1016/j.molcel.2025.07.012. Epub 2025 Aug 12. Mol Cell. 2025. PMID: 40803327
-
Epigenetic Regulation of Erythropoiesis: From Developmental Programs to Therapeutic Targets.Int J Mol Sci. 2025 Jun 30;26(13):6342. doi: 10.3390/ijms26136342. Int J Mol Sci. 2025. PMID: 40650116 Free PMC article. Review.
-
Pre-existing immunity does not impair the engraftment of CRISPR-Cas9-edited cells in rhesus macaques conditioned with busulfan or radiation.Mol Ther Methods Clin Dev. 2023 Apr 20;29:483-493. doi: 10.1016/j.omtm.2023.04.004. eCollection 2023 Jun 8. Mol Ther Methods Clin Dev. 2023. PMID: 37273902 Free PMC article.
References
-
- Rao S.S.P., Huntley M.H., Durand N.C., Stamenova E.K., Bochkov I.D., Robinson J.T., Sanborn A.L., Machol I., Omer A.D., Lander E.S., Aiden E.L. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

