Molecular oxygen as a probe molecule in EPR spin-labeling studies of membrane structure and dynamics
- PMID: 36852103
- PMCID: PMC9965258
- DOI: 10.3390/oxygen2030021
Molecular oxygen as a probe molecule in EPR spin-labeling studies of membrane structure and dynamics
Abstract
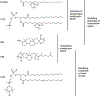
Molecular oxygen (O2) is the perfect probe molecule for membrane studies carried out using the saturation recovery EPR technique. O2 is a small, paramagnetic, hydrophobic enough molecule that easily partitions into a membrane's different phases and domains. In membrane studies, the saturation recovery EPR method requires two paramagnetic probes: a lipid-analog nitroxide spin label and an oxygen molecule. The experimentally derived parameters of this method are the spin-lattice relaxation times (T 1s) of spin labels and rates of bimolecular collisions between O2 and the nitroxide fragment. Thanks to the long T 1 of lipid spin labels (from 1 to 10 μs), the approach is very sensitive to changes of the local (around the nitroxide fragment) O2 diffusion-concentration product. Small variations in the lipid packing affect O2 solubility and O2 diffusion, which can be detected by the shortening of T 1 of spin labels. Using O2 as a probe molecule and a different lipid spin label inserted into specific phases of the membrane and membrane domains allows data about the lateral arrangement of lipid membranes to be obtained. Moreover, using a lipid spin label with the nitroxide fragment attached to its head group or a hydrocarbon chain at different positions also enables data about molecular dynamics and structure at different membrane depths to be obtained. Thus, the method can be used to investigate not only the lateral organization of the membrane (i.e., the presence of membrane domains and phases), but also the depth-dependent membrane structure and dynamics, and, hence, the membrane properties in three dimensions.
Keywords: EPR; Molecular oxygen; cholesterol; lipid bilayer membranes; lipid spin labels; membrane domains; membrane fluidity.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflict of interest.
Figures






Similar articles
-
Saturation-recovery electron paramagnetic resonance discrimination by oxygen transport (DOT) method for characterizing membrane domains.Methods Mol Biol. 2007;398:143-57. doi: 10.1007/978-1-59745-513-8_11. Methods Mol Biol. 2007. PMID: 18214379
-
Differences in the properties of porcine cortical and nuclear fiber cell plasma membranes revealed by saturation recovery EPR spin labeling measurements.Exp Eye Res. 2021 May;206:108536. doi: 10.1016/j.exer.2021.108536. Epub 2021 Mar 12. Exp Eye Res. 2021. PMID: 33716012 Free PMC article.
-
Spin-Lattice Relaxation Rates of Lipid Spin Labels as a Measure of Their Rotational Diffusion Rates in Lipid Bilayer Membranes.Membranes (Basel). 2022 Sep 30;12(10):962. doi: 10.3390/membranes12100962. Membranes (Basel). 2022. PMID: 36295720 Free PMC article.
-
Multilamellar Liposomes as a Model for Biological Membranes: Saturation Recovery EPR Spin-Labeling Studies.Membranes (Basel). 2022 Jun 26;12(7):657. doi: 10.3390/membranes12070657. Membranes (Basel). 2022. PMID: 35877860 Free PMC article. Review.
-
Oxygenic photosynthesis: EPR study of photosynthetic electron transport and oxygen-exchange, an overview.Cell Biochem Biophys. 2019 Mar;77(1):47-59. doi: 10.1007/s12013-018-0861-6. Epub 2018 Nov 20. Cell Biochem Biophys. 2019. PMID: 30460441 Free PMC article. Review.
Cited by
-
5-PC as a Lipid Probe Molecule and as a Second Phospholipid in Binary Phospholipid Mixtures: Saturation Recovery EPR Studies.Int J Mol Sci. 2024 Nov 30;25(23):12913. doi: 10.3390/ijms252312913. Int J Mol Sci. 2024. PMID: 39684621 Free PMC article.
-
How Do Xanthophylls Protect Lipid Membranes from Oxidative Damage?J Phys Chem Lett. 2023 Aug 24;14(33):7440-7444. doi: 10.1021/acs.jpclett.3c01374. Epub 2023 Aug 14. J Phys Chem Lett. 2023. PMID: 37578906 Free PMC article.
-
Quantification of Age-Related Changes in the Lateral Organization of the Lipid Portion of the Intact Membranes Isolated from the Left and Right Eye Lenses of the Same Human Donor.Membranes (Basel). 2023 Feb 3;13(2):189. doi: 10.3390/membranes13020189. Membranes (Basel). 2023. PMID: 36837692 Free PMC article.
-
Cholesterol inhibits oxygen permeation through biological membranes: mechanism against double-bond peroxidation.RSC Adv. 2024 Sep 13;14(40):29113-29121. doi: 10.1039/d4ra04846f. eCollection 2024 Sep 12. RSC Adv. 2024. PMID: 39282058 Free PMC article.
-
An Overview of Lutein in the Lipid Membrane.Int J Mol Sci. 2023 Aug 18;24(16):12948. doi: 10.3390/ijms241612948. Int J Mol Sci. 2023. PMID: 37629129 Free PMC article. Review.
References
-
- Danielli JF and Davson H, A contribution to the theory of permeability of thin films. J Cell Physiol, 1935, 5, p. 495–508.
-
- Singer SJ and Nicolson GL, The fluid mosaic model of the structure of cell membranes. Science, 1972, 175, p. 720–31. - PubMed
-
- Robertson RN, The lively membranes, Cambridge University Press, 1983.
-
- Kusumi A, et al., Hierarchical mesoscale domain organization of the plasma membrane. Trends Biochem Sci, 2011, 36, p. 604–15. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
