Nanocellulose composite wound dressings for real-time pH wound monitoring
- PMID: 36852226
- PMCID: PMC9958357
- DOI: 10.1016/j.mtbio.2023.100574
Nanocellulose composite wound dressings for real-time pH wound monitoring
Abstract
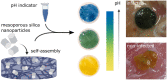
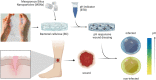
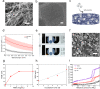
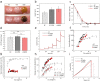
The skin is the largest organ of the human body. Wounds disrupt the functions of the skin and can have catastrophic consequences for an individual resulting in significant morbidity and mortality. Wound infections are common and can substantially delay healing and can result in non-healing wounds and sepsis. Early diagnosis and treatment of infection reduce risk of complications and support wound healing. Methods for monitoring of wound pH can facilitate early detection of infection. Here we show a novel strategy for integrating pH sensing capabilities in state-of-the-art hydrogel-based wound dressings fabricated from bacterial nanocellulose (BC). A high surface area material was developed by self-assembly of mesoporous silica nanoparticles (MSNs) in BC. By encapsulating a pH-responsive dye in the MSNs, wound dressings for continuous pH sensing with spatiotemporal resolution were developed. The pH responsive BC-based nanocomposites demonstrated excellent wound dressing properties, with respect to conformability, mechanical properties, and water vapor transmission rate. In addition to facilitating rapid colorimetric assessment of wound pH, this strategy for generating functional BC-MSN nanocomposites can be further be adapted for encapsulation and release of bioactive compounds for treatment of hard-to-heal wounds, enabling development of novel wound care materials.
Keywords: Bacterial nanocellulose; Infection; Mesoporous silica nanoparticles; Wound dressing; pH sensor.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Haesler E., Ousey K., Lecturer H.S., Associate H., Prevention I. Clinical practice Evolution of the wound infection continuum. Wounds Int. 2018;9:6–10.
-
- Singer M., Deutschman C.S., Seymour C., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., Der Poll T., Vincent J.L., Angus D.C. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA, J. Am. Med. Assoc. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Huang C.Y., Daniels R., Lembo A., Hartog C., O'Brien J., Heymann T., Reinhart K., Nguyen H.B., Azevedo L., Finfer S., Fleischmann C., Foreman T., Kissoon N., Machado F., Martens C., Possagnoli I., Hanke R., Vroomen-Durning M. Life after sepsis: an international survey of survivors to understand the post-sepsis syndrome. Int. J. Qual. Health Care. 2019;31:191–198. doi: 10.1093/intqhc/mzy137. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

