Using biological constraints to improve prediction in precision oncology
- PMID: 36852282
- PMCID: PMC9958363
- DOI: 10.1016/j.isci.2023.106108
Using biological constraints to improve prediction in precision oncology
Abstract
Many gene signatures have been developed by applying machine learning (ML) on omics profiles, however, their clinical utility is often hindered by limited interpretability and unstable performance. Here, we show the importance of embedding prior biological knowledge in the decision rules yielded by ML approaches to build robust classifiers. We tested this by applying different ML algorithms on gene expression data to predict three difficult cancer phenotypes: bladder cancer progression to muscle-invasive disease, response to neoadjuvant chemotherapy in triple-negative breast cancer, and prostate cancer metastatic progression. We developed two sets of classifiers: mechanistic, by restricting the training to features capturing specific biological mechanisms; and agnostic, in which the training did not use any a priori biological information. Mechanistic models had a similar or better testing performance than their agnostic counterparts, with enhanced interpretability. Our findings support the use of biological constraints to develop robust gene signatures with high translational potential.
Keywords: Cancer; Machine learning; Omics; Precision medicine.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

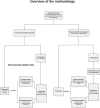

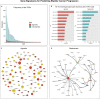

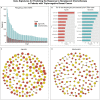
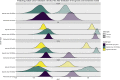
References
-
- Knezevic D., Goddard A.D., Natraj N., Cherbavaz D.B., Clark-Langone K.M., Snable J., Watson D., Falzarano S.M., Magi-Galluzzi C., Klein E.A., Quale C. Analytical validation of the Oncotype DX prostate cancer assay - a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genom. 2013;14:690. doi: 10.1186/1471-2164-14-690. - DOI - PMC - PubMed
-
- Keogh E., Mueen A. Encyclopedia of machine learning; 2010. Curse of Dimensionality; pp. 257–258.
-
- Hand D.J. Classifier technology and the illusion of progress. Stat. Sci. 2006;21:1–14. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

