IgE-immunoadsorption for severe allergy to multiple foods: A case series of five children
- PMID: 36852410
- PMCID: PMC9958481
- DOI: 10.1016/j.waojou.2023.100750
IgE-immunoadsorption for severe allergy to multiple foods: A case series of five children
Abstract
Background: Children with severe food allergy may present high risk of fatal anaphylaxis and a highly impaired quality of life. Anti IgE-treatment has been shown to be a promising approach as monotherapy for severe allergy to multiple foods. However, very high serum total IgE levels may limit its use.This study aims to assess the efficacy of IgE-selective immunoadsorption (IgE-IA) on total IgE levels and threshold of reactivity to the culprit foods in children with history of severe anaphylaxis due to multiple foods and allergic comorbidities.
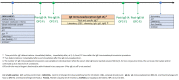
Methods: In this single-center, prospective, open-label efficacy study we evaluated children with severe asthma, allergy to 2+foods and total IgE levels >2300 kUI/L. To establish the food reactivity threshold, each patient underwent oral food challenges (OFCs) before and after IgE-IA.
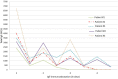
Results: Five patients (4 males; age, 12.2 ± 5 years, mean ± SD) underwent an average of 3 (range 2-4) sessions of IgE-IA. Each session reduced IgE levels by a mean of 1958.87 kUI/L. After the IgE-IA cycle, serum total IgE dropped from 3948 ± 1652.7 (mean ± SD) to 360.8 ± 71.9 kUI/L (-10.9 folds; p = 0.01). The threshold of reactivity (No Observed Adverse Effect Level, NOAEL) tested at OFCs for the culprit foods (4 baked-milk + 2 baked-egg + 1 lentil + 2 hazelnut + 1 wheat) increased overall from 21.5 (median, IQR 1.5-82.6) protein milligrams to 1115 (837.2-4222.8) milligrams (p < 0.001), ie, up to 51.8 times higher than baseline. 8/10 OFCs were negative after IgE-IA.
Conclusions: IgE-IA increased food threshold quickly. It can be considered in well-selected patients with severe food allergies and high IgE-levels especially if otherwise eligible to anti IgE treatment.
Keywords: Anti-IgE treatment; Children; IgE-immunoadsorption; Omalizumab; Severe food allergy.
© 2023 The Author(s).
Figures

References
LinkOut - more resources
Full Text Sources

