Srs11-92, a ferrostatin-1 analog, improves oxidative stress and neuroinflammation via Nrf2 signal following cerebral ischemia/reperfusion injury
- PMID: 36852441
- PMCID: PMC10173707
- DOI: 10.1111/cns.14130
Srs11-92, a ferrostatin-1 analog, improves oxidative stress and neuroinflammation via Nrf2 signal following cerebral ischemia/reperfusion injury
Abstract
Aim: Ferroptosis is increasingly becoming to be considered as an important mechanism of pathological cell death during stroke, and specific exogenous ferroptosis inhibitors have the ability to reverse cerebral ischemia/reperfusion injury. However, research on Srs11-92 (AA9), a ferrostatin-1 (Fer-1) analog, in preclinical studies is limited.
Methods: In the middle cerebral artery occlusion-reperfusion (MCAO/R) mice model or oxygen-glucose deprivation/reperfusion (OGD/R) cell model, Fer-1, AA9, and/or ML385 were administered, and brain infarct size, neurological deficits, neuronal damage, oxidative stress, and neuroinflammation were determined after the damage, in vitro and in vivo.
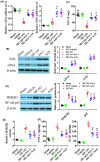
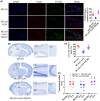
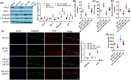
Results: Fer-1 and AA9 improved brain infarct size, neuronal damage, and neurological deficits in mice model of MCAO/R, and inhibited the overloaded iron deposition, ROS accumulation, and neuroinflammation response: it also increased the expression of GPx4, Nrf2, and HO-1 and suppressed the expression of HMGB1 and NF-κB p65 in the epicenter of injured hippocampal formation. However, Nrf2 inhibitor ML385 reversed the neuroprotective effect of AA9, including the oxidative stress and neuroinflammation. In vitro studies showed that AA9 relieved OGD/R-induced neuronal oxidative stress and neuroinflammation via the Nrf2 pathway, which was impaired by ML385 in primary neurons.
Conclusion: The findings imply that Fer-1 analog AA9 may be suitable for further translational studies for the protection of neuronal damage via Nrf2 signal pathway-mediated oxidative stress and neuroinflammation in stroke and others neurological diseases.
Keywords: Srs11-92; ferroptosis; ischemic stroke; neuroinflammation; oxidative stress.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke Statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254‐e743. - PubMed
-
- Datta A, Sarmah D, Mounica L, et al. Cell death pathways in ischemic stroke and targeted pharmacotherapy. Transl Stroke Res. 2020;11(6):1185‐1202. - PubMed
-
- Hughes RE, Tadi P, Bollu PC. TPA therapy. StatPearls. StatPearls Publishing; 2021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

