Vascular stability of brain arteriovenous malformations after partial embolization
- PMID: 36852445
- PMCID: PMC10915995
- DOI: 10.1111/cns.14136
Vascular stability of brain arteriovenous malformations after partial embolization
Abstract
Introduction: Brain arteriovenous malformation (bAVM) might have a higher risk of rupture after partial embolization, and previous studies have shown that some metrics of vascular stability are related to bAVM rupture risk.
Objective: To analyze vascular stability of bAVM in patients after partial embolization.
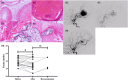
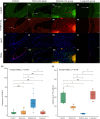
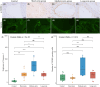
Methods: Twenty-four patients who underwent partial embolization were classified into the short-term, medium-term, and long-term groups, according to the time interval between partial embolization and surgery. The control group consisted of 9 bAVM patients who underwent surgery alone. Hemodynamic changes after partial embolization were measured by angiogram. The inflammatory infiltrates and cell-cell junctions were evaluated by MMP-9 and VE-cadherin. At the protein level, the proliferative and apoptotic events of bAVMs were analyzed by immunohistochemical staining of VEGFA, eNOS, and caspase-3. Finally, neovascularity and apoptotic cells were assessed by CD31 staining and TUNEL staining.
Results: Immediately after partial embolization, the blood flow velocity of most bAVMs increased. The quantity of MMP-9 in the medium-term group was the highest, and VE-cadherin in the medium-term group was the lowest. The expression levels of VEGFA, eNOS, and neovascularity were highest in the medium-term group. Similarly, the expression level of caspase-3 and the number of apoptotic cells were highest in the medium-term group.
Conclusion: The biomarkers for bAVM vascular stability were most abnormal between 1 and 28 days after partial embolization.
Keywords: angiogenesis; brain arteriovenous malformation; intracerebral hemorrhage; partial embolization.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
None.
Figures

References
-
- Chen CJ, Ding D, Derdeyn CP, et al. Brain arteriovenous malformations: a review of natural history, pathobiology, and interventions. Neurology. 2020;95(20):917‐927. - PubMed
-
- Nerva JD, Kim LJ, Barber J, et al. Outcomes of multimodality therapy in pediatric patients with ruptured and unruptured brain arteriovenous malformations. Neurosurgery. 2016;78(5):695‐707. - PubMed
-
- Sure U, Butz N, Siegel AM, Mennel HD, Bien S, Bertalanffy H. Treatment‐induced neoangiogenesis in cerebral arteriovenous malformations. Clin Neurol Neurosurg. 2001;103(1):29‐32. - PubMed
-
- Lv X, Wu Z, Li Y, Yang X, Jiang C. Hemorrhage risk after partial endovascular NBCA and ONYX embolization for brain arteriovenous malformation. Neurol Res. 2012;34(6):552‐556. - PubMed
-
- Laakso A, Dashti R, Seppänen J, et al. Long‐term excess mortality in 623 patients with brain arteriovenous malformations. Neurosurgery. 2008;63(2):244‐253. discussion 253–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

