Hypermethylation-Mediated lncRNA MAGI2-AS3 Downregulation Facilitates Malignant Progression of Laryngeal Squamous Cell Carcinoma via Interacting With SPT6
- PMID: 36852700
- PMCID: PMC9986895
- DOI: 10.1177/09636897231154574
Hypermethylation-Mediated lncRNA MAGI2-AS3 Downregulation Facilitates Malignant Progression of Laryngeal Squamous Cell Carcinoma via Interacting With SPT6
Abstract
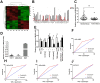
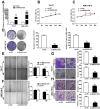
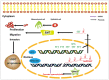
Long noncoding RNAs (lncRNAs) have an effect on the occurrence and progression of a considerable number of diseases, especially cancer. Existing research has suggested that MAGI2 antisense RNA 3 (MAGI2-AS3) takes on a critical significance in the development of hepatocellular carcinoma and lung cancer. However, the functions of MAGI2-AS3 in laryngeal squamous cell carcinoma (LSCC) remain unclear. In this study, MAGI2-AS3 expression level in LSCC tissue and cell lines was detected, and the effect of MAGI2-AS3 overexpressed on LSCC phenotypes and the possible influence mechanisms were examined. MAGI2-AS3 was downregulated in the tissues of LSCC patients versus non-tumor tissues, and it was correlated with advanced TNM (tumor, node, metastasis) stage and lymph node metastases, as indicated by the results of this study. MAGI2-AS3 inhibited the proliferation, migration, and invasion of LSCC cells in vitro and in vivo. Furthermore, the hypermethylation level of the MAGI2-AS3 promoter region was indicated by bisulfite genomic sequencing and methylation-specific polymerase chain reaction, such that MAGI2-AS3 expression was downregulated. Besides, MAGI2-AS3 promoter hypermethylation was regulated by DNA methyltransferase 1 (DNMT1), and MAGI2-AS3 expression was reversed by 5-Aza-2'-deoxycytidine (5-Aza). Moreover, the result of the RNA pull-down experiment suggested that 38 proteins were enriched in the MAGI2-AS3 group versus the control group in TU177 cells. To be specific, SPT6 (ie, a conserved protein) was enriched by fold change >10. SPT6 knockdown reduced the antitumor effect of MAGI2-AS3 in TU177 and AMC-HN-8 cells. Meanwhile, SPT6 overexpression inhibited the proliferation, metastasis, and invasion of TU177 and AMC-HN-8 cells. As revealed by the above findings, DNMT1-regulated MAGI2-AS3 promoter hypermethylation led to downregulated MAGI2-AS3 expression, such that the presence and progression of LSCC were inhibited in an SPT6 binding-dependent manner.
Keywords: DNA methylation; MAGI2-AS3; SPT6; biomarker; laryngeal squamous cell carcinoma.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

