Structural features stabilized by divalent cation coordination within hepatitis E virus ORF1 are critical for viral replication
- PMID: 36852909
- PMCID: PMC9977285
- DOI: 10.7554/eLife.80529
Structural features stabilized by divalent cation coordination within hepatitis E virus ORF1 are critical for viral replication
Abstract
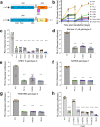
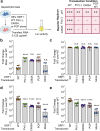
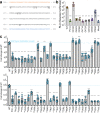
Hepatitis E virus (HEV) is an RNA virus responsible for over 20 million infections annually. HEV's open reading frame (ORF)1 polyprotein is essential for genome replication, though it is unknown how the different subdomains function within a structural context. Our data show that ORF1 operates as a multifunctional protein, which is not subject to proteolytic processing. Supporting this model, scanning mutagenesis performed on the putative papain-like cysteine protease (pPCP) domain revealed six cysteines essential for viral replication. Our data are consistent with their role in divalent metal ion coordination, which governs local and interdomain interactions that are critical for the overall structure of ORF1; furthermore, the 'pPCP' domain can only rescue viral genome replication in trans when expressed in the context of the full-length ORF1 protein but not as an individual subdomain. Taken together, our work provides a comprehensive model of the structure and function of HEV ORF1.
Keywords: hepatitis E virus; infectious disease; microbiology; molecular biophysics; replication; structural biology; viral hepatitis; virus infection; viruses.
© 2023, LeDesma et al.
Conflict of interest statement
RL, BH, AB, SM, SG, JH, AP No competing interests declared
Figures


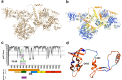



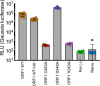

Comment in
-
Learning more about hepatitis E virus.Elife. 2023 Mar 22;12:e87047. doi: 10.7554/eLife.87047. Elife. 2023. PMID: 36947136 Free PMC article.
References
-
- Ansari IH, Nanda SK, Durgapal H, Agrawal S, Mohanty SK, Gupta D, Jameel S, Panda SK. Cloning, sequencing, and expression of the hepatitis E virus (HEV) nonstructural open reading frame 1 (ORF1) Journal of Medical Virology. 2000;60:275–283. doi: 10.1002/(SICI)1096-9071(200003)60:3<275::AID-JMV5>3.0.CO;2-9. - DOI - PubMed
-
- Bergmann EM, Cherney MM, Mckendrick J, Frormann S, Luo C, Malcolm BA, Vederas JC, James MN. Crystal structure of an inhibitor complex of the 3C proteinase from hepatitis A virus (HAV) and implications for the polyprotein processing in HAV. Virology. 1999;265:153–163. doi: 10.1006/viro.1999.9968. - DOI - PubMed
-
- Boeckmann B, Bairoch A, Apweiler R, Blatter MC, Estreicher A, Gasteiger E, Martin MJ, Michoud K, O’Donovan C, Phan I, Pilbout S, Schneider M. The SWISS-PROT protein knowledgebase and its supplement trembl in 2003. Nucleic Acids Research. 2003;31:365–370. doi: 10.1093/nar/gkg095. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

