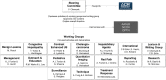
LI-RADS: Looking Back, Looking Forward
- PMID: 36853182
- PMCID: PMC10068888
- DOI: 10.1148/radiol.222801
LI-RADS: Looking Back, Looking Forward
Abstract
Since its initial release in 2011, the Liver Imaging Reporting and Data System (LI-RADS) has evolved and expanded in scope. It started as a single algorithm for hepatocellular carcinoma (HCC) diagnosis with CT or MRI with extracellular contrast agents and has grown into a multialgorithm network covering all major liver imaging modalities and contexts of use. Furthermore, it has developed its own lexicon, report templates, and supplementary materials. This article highlights the major achievements of LI-RADS in the past 11 years, including adoption in clinical care and research across the globe, and complete unification of HCC diagnostic systems in the United States. Additionally, the authors discuss current gaps in knowledge, which include challenges in surveillance, diagnostic population definition, perceived complexity, limited sensitivity of LR-5 (definite HCC) category, management implications of indeterminate observations, challenges in reporting, and treatment response assessment following radiation-based therapies and systemic treatments. Finally, the authors discuss future directions, which will focus on mitigating the current challenges and incorporating advanced technologies. Tha authors envision that LI-RADS will ultimately transform into a probability-based system for diagnosis and prognostication of liver cancers that will integrate patient characteristics and quantitative imaging features, while accounting for imaging modality and contrast agent.
© RSNA, 2023.
Conflict of interest statement
Figures





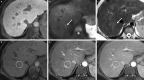
![CT images show examples of observations that meet criteria for Liver
Imaging Reporting and Data System (LI-RADS) category LR-M (probably or
definitely malignant, not hepatocellular carcinoma [HCC] specific). Axial
contrast-enhanced CT in (A) arterial phase and (B) portal venous phase in a
56-year-old woman with nonalcoholic steatohepatitis-induced cirrhosis
demonstrates a 36-mm observation (arrow, B) with rim arterial phase
hyperenhancement (arrow, A). Pathology revealed intrahepatic
cholangiocarcinoma. Axial contrast-enhanced CT in (C) arterial phase and (D)
portal venous phase in a 63-year-old man with hepatitis C virus cirrhosis
demonstrates a 90-mm observation (arrow, D) with rim arterial phase
hyperenhancement (arrow, C). Pathology revealed poorly differentiated HCC
with p53 mutation.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/b9f2/10068888/acbd0f3a2a67/radiol.222801.fig7.gif)


References
-
- Marrero JA , Kulik LM , Sirlin CB , et al . Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases . Hepatology 2018. ; 68 ( 2 ): 723 – 750 . - PubMed
-
- Tang A , Cruite I , Mitchell DG , Sirlin CB . Hepatocellular carcinoma imaging systems: why they exist, how they have evolved, and how they differ . Abdom Radiol (NY) 2018. ; 43 ( 1 ): 3 – 12 . - PubMed
-
- Liver Reporting and Data System . American College of Radiology . https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RAD... . Accessed January 31, 2023.
-
- Sirlin CB . The LI-RADS adventure-a personal statement . Abdom Radiol (NY) 2018. ; 43 ( 1 ): 1 – 2 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

