Outcomes of intraventricular 131-I-omburtamab and external beam radiotherapy in patients with recurrent medulloblastoma and ependymoma
- PMID: 36853490
- PMCID: PMC10050019
- DOI: 10.1007/s11060-022-04235-w
Outcomes of intraventricular 131-I-omburtamab and external beam radiotherapy in patients with recurrent medulloblastoma and ependymoma
Abstract
Purpose: Intraventricular compartmental radioimmunotherapy (cRIT) with 131-I-omburtamab is a potential therapy for recurrent primary brain tumors that can seed the thecal space. These patients often previously received external beam radiotherapy (EBRT) to a portion or full craniospinal axis (CSI) as part of upfront therapy. Little is known regarding outcomes after re-irradiation as part of multimodality therapy including cRIT. This study evaluates predictors of response, patterns of failure, and radiologic events after cRIT.
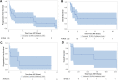
Methods: Patients with recurrent medulloblastoma or ependymoma who received 131-I-omburtamab on a prospective clinical trial were included. Extent of disease at cRIT initiation (no evidence of disease [NED] vs measurable disease [MD]) was assessed as associated with progression-free (PFS) and overall survival (OS) by Kaplan-Meier analysis.
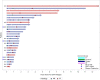
Results: All 27 patients (20 medulloblastoma, 7 ependymoma) had EBRT preceding cRIT: most (22, 81%) included CSI (median dose 2340 cGy, boost to 5400 cGy). Twelve (44%) also received EBRT at relapse as bridging to cRIT. There were no cases of radionecrosis. At cRIT initiation, 11 (55%) medulloblastoma and 3 (43%) ependymoma patients were NED, associated with improved PFS (p = 0.002) and OS (p = 0.048) in medulloblastoma. Most relapses were multifocal. With medium follow-up of 3.0 years (95% confidence interval, 1.8-7.4), 6 patients remain alive with NED.
Conclusion: For patients with medulloblastoma, remission at time of cRIT was associated with significantly improved survival outcomes. Relapses are often multifocal, particularly in the setting of measurable disease at cRIT initiation. EBRT is a promising tool to achieve NED status at cRIT initiation, with no cases of radiation necrosis.
Keywords: External beam radiotherapy; Intraventricular compartmental radioimmunotherapy; Pediatric brain tumors; Proton beam radiotherapy; Radiation necrosis.
© 2023. The Author(s).
Conflict of interest statement
Both Memorial Sloan Kettering Cancer Center (MSK) and Dr. Cheung have financial interest in Y-mAbs, Abpro-Labs and Eureka Therapeutics. Dr. Cheung also reports receiving commercial research grants from Y-mabs Therapeutics and Abpro-Labs Inc. Dr. Cheung was named as inventor on multiple patents filed by MSK, including naxitamab and omburtamab licensed to Ymabs Therapeutics, and others licensed to Biotec Pharmacon, and Abpro-labs. Dr. Cheung is a SAB member for Eureka Therapeutics. Dr. Modak and Dr. Kramer reports consulting and equity in Ymabs, Therapeutics Inc. Omburtamab was licensed to Y-mAbs Therapeutics in 2015. Dr. Karajannis reports grants from Y-mAbs Therapeutics, Inc. (research support) and personal fees from Bayer AG, AstraZeneca, Inc., QED Therapeutics, Inc., CereXis, Inc., Recursion, Inc., Cardinal Health and Medscape (Medical Advisory Board and/or consultant).
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

