Association of Vitamin C, Thiamine, and Hydrocortisone Infusion With Long-term Cognitive, Psychological, and Functional Outcomes in Sepsis Survivors: A Secondary Analysis of the Vitamin C, Thiamine, and Steroids in Sepsis Randomized Clinical Trial
- PMID: 36853612
- PMCID: PMC9975932
- DOI: 10.1001/jamanetworkopen.2023.0380
Association of Vitamin C, Thiamine, and Hydrocortisone Infusion With Long-term Cognitive, Psychological, and Functional Outcomes in Sepsis Survivors: A Secondary Analysis of the Vitamin C, Thiamine, and Steroids in Sepsis Randomized Clinical Trial
Erratum in
-
Error in the Byline.JAMA Netw Open. 2023 Apr 3;6(4):e2312173. doi: 10.1001/jamanetworkopen.2023.12173. JAMA Netw Open. 2023. PMID: 37079308 Free PMC article. No abstract available.
Abstract
Importance: Sepsis is associated with long-term cognitive impairment and worse psychological and functional outcomes. Potential mechanisms include intracerebral oxidative stress and inflammation, yet little is known about the effects of early antioxidant and anti-inflammatory therapy on cognitive, psychological, and functional outcomes in sepsis survivors.
Objective: To describe observed differences in long-term cognitive, psychological, and functional outcomes of vitamin C, thiamine, and hydrocortisone between the intervention and control groups in the Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) randomized clinical trial.
Design, setting, and participants: This prespecified secondary analysis reports the 6-month outcomes of the multicenter, double-blind, placebo-controlled VICTAS randomized clinical trial, which was conducted between August 2018 and July 2019. Adult patients with sepsis-induced respiratory and/or cardiovascular dysfunction who survived to discharge or day 30 were recruited from 43 intensive care units in the US. Participants were randomized 1:1 to either the intervention or control group. Cognitive, psychological, and functional outcomes at 6 months after randomization were assessed via telephone through January 2020. Data analyses were conducted between February 2021 and December 2022.
Interventions: The intervention group received intravenous vitamin C (1.5 g), thiamine hydrochloride (100 mg), and hydrocortisone sodium succinate (50 mg) every 6 hours for 96 hours or until death or intensive care unit discharge. The control group received matching placebo.
Main outcomes and measures: Cognitive performance, risk of posttraumatic stress disorder and depression, and functional status were assessed using a battery of standardized instruments that were administered during a 1-hour telephone call 6 months after randomization.
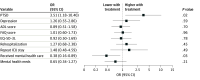
Results: After exclusions, withdrawals, and deaths, the final sample included 213 participants (median [IQR] age, 57 [47-67] years; 112 males [52.6%]) who underwent long-term outcomes assessment and had been randomized to either the intervention group (n = 108) or control group (n = 105). The intervention group had lower immediate memory scores (adjusted OR [aOR], 0.49; 95% CI, 0.26-0.89), higher odds of posttraumatic stress disorder (aOR, 3.51; 95% CI, 1.18-10.40), and lower odds of receiving mental health care (aOR, 0.38; 95% CI, 0.16-0.89). No other statistically significant differences in cognitive, psychological, and functional outcomes were found between the 2 groups.
Conclusions and relevance: In survivors of sepsis, treatment with vitamin C, thiamine, and hydrocortisone did not improve or had worse cognitive, psychological, and functional outcomes at 6 months compared with patients who received placebo. These findings challenge the hypothesis that antioxidant and anti-inflammatory therapy during critical illness mitigates the development of long-term cognitive, psychological, and functional impairment in sepsis survivors.
Trial registration: ClinicalTrials.gov Identifier: NCT03509350.
Conflict of interest statement
Figures

Comment in
-
Critical Care and the Postintensive Care Syndrome.JAMA Netw Open. 2023 Feb 1;6(2):e230391. doi: 10.1001/jamanetworkopen.2023.0391. JAMA Netw Open. 2023. PMID: 36853614 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

