Mechanical conditioning of human mesenchymal stem cells for enhancing vascular regeneration
- PMID: 36853695
- PMCID: PMC9929623
- DOI: 10.1016/j.xpro.2023.102103
Mechanical conditioning of human mesenchymal stem cells for enhancing vascular regeneration
Abstract
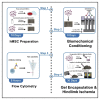
Human mesenchymal stem cells (hMSCs) are an appealing cell type for therapeutic applications but remain limited by poor efficacy in clinical trials. Here, we describe a conditioning technique that enhances the vascular regenerative properties of hMSCs and increases their expression of endothelial cell and pericyte markers. We also describe an alginate gel encapsulation protocol for delivering the conditioned cells. For complete details on the use and execution of this protocol, please refer to Lee et al. (2021).1.
Keywords: Biotechnology and Bioengineering; Cell Biology; Cell Culture; Flow Cytometry/Mass Cytometry; Health Sciences; Model Organisms; Stem Cells; Tissue Engineering.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.B.B. and J.L. have a patent on the technology described in this work (US Patent US20200268801A1).
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

