A Monoclonal Antibody That Provides a Model for C3 Nephritic Factors
- PMID: 36853837
- PMCID: PMC9983123
- DOI: 10.1089/mab.2022.0028
A Monoclonal Antibody That Provides a Model for C3 Nephritic Factors
Abstract
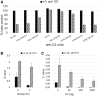
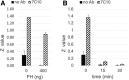
Complement is a major innate defense system that protects the intravascular space from microbial invasion. Complement activation results in the assembly of C3 convertases, serine proteases that cleave complement protein C3, generating bioactive fragments C3a and C3b. The complement response is rapid and robust, largely due to a positive feedback regulatory loop mediated by alternative pathway (AP) C3 convertase. C3 nephritic factors (C3NEFs) are autoantibodies that stabilize AP convertase, resulting in uncontrolled C3 cleavage, which, in principle, can promote critical tissue injury similar to that seen in certain renal conditions. Investigations of C3NEFs are hampered by a challenging issue: each C3NEF is derived from a different donor source, and there is no method to compare one C3NEF to another. We have identified a widely available mouse anti-C3 mAb that, similar to many C3NEFs, can stabilize functional AP convertase in a form resistant to decay acceleration by multiple complement regulators. The antibody requires the presence of properdin to confer convertase stability, and hampers the activity of Salp20, a tic salivary protein that accelerates convertase dissociation by displacing properdin from the convertase complex. This mAb can serve as an urgently needed standard for the investigation of C3NEFs. This study also provides novel insights into the dynamics of AP convertase.
Keywords: C3 glomerulonephritis; C3 nephritic factor; alternative pathway; complement; convertase; properdin.
Conflict of interest statement
No competing financial interests exist.
Figures


References
-
- Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science 1996;272:50–54. - PubMed
-
- Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol 2004;5(10):981–986. - PubMed
-
- Kemper C, Atkinson JP. T-cell regulation: With complements from innate immunity. Nat Rev Immunol 2007;7(1):9–18. - PubMed
-
- Ollert MW, Kadlec JV, David K, et al. . Antibody-mediated complement activation on nucleated cells. A quantitative analysis of the individual reaction steps. J Immunol 1994;153(5):2213–2221. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

