Metastasis from the tumor interior and necrotic core formation are regulated by breast cancer-derived angiopoietin-like 7
- PMID: 36853945
- PMCID: PMC10013750
- DOI: 10.1073/pnas.2214888120
Metastasis from the tumor interior and necrotic core formation are regulated by breast cancer-derived angiopoietin-like 7
Abstract
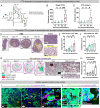
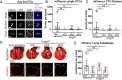
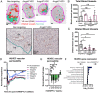
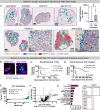
Necrosis in the tumor interior is a common feature of aggressive cancers that is associated with poor clinical prognosis and the development of metastasis. How the necrotic core promotes metastasis remains unclear. Here, we report that emergence of necrosis inside the tumor is correlated temporally with increased tumor dissemination in a rat breast cancer model and in human breast cancer patients. By performing spatially focused transcriptional profiling, we identified angiopoietin-like 7 (Angptl7) as a tumor-specific factor localized to the perinecrotic zone. Functional studies showed that Angptl7 loss normalizes central necrosis, perinecrotic dilated vessels, metastasis, and reduces circulating tumor cell counts to nearly zero. Mechanistically, Angptl7 promotes vascular permeability and supports vascular remodeling in the perinecrotic zone. Taken together, these findings show that breast tumors actively produce factors controlling central necrosis formation and metastatic dissemination from the tumor core.
Keywords: angiopoietin-like 7; breast cancer; circulating tumor cells; metastasis; necrotic core.
Conflict of interest statement
A.Y., Y.H., B.A.K., and K.J.C. are inventors on a pending patent application related to this work.
Figures


References
-
- Friedl P., Wolf K., Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 3, 362–374 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

