Emergent variant modeling of the serological repertoire to norovirus in young children
- PMID: 36854303
- PMCID: PMC10040388
- DOI: 10.1016/j.xcrm.2023.100954
Emergent variant modeling of the serological repertoire to norovirus in young children
Abstract
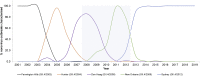
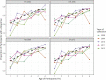
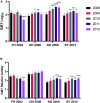
Human norovirus is the leading cause of acute gastroenteritis. Young children and the elderly bear the greatest burden of disease, representing more than 200,000 deaths annually. Infection prevalence peaks at younger than 2 years and is driven by novel GII.4 variants that emerge and spread globally. Using a surrogate neutralization assay, we characterize the evolution of the serological neutralizing antibody (nAb) landscape in young children as they transition between sequential GII.4 pandemic variants. Following upsurge of the replacement variant, antigenic cartography illustrates remodeling of the nAb landscape to the new variant accompanied by improved nAb titer. However, nAb relative avidity remains focused on the preceding variant. These data support immune imprinting as a mechanism of immune evasion and GII.4 virus persistence across a population. Understanding the complexities of immunity to rapidly evolving and co-circulating viral variants, like those of norovirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), and dengue viruses, will fundamentally inform vaccine design for emerging pathogens.
Keywords: antigenic cartography; antigenic seniority; blockade antibodies; immune imprinting; neutralizing antibodies; norovirus; variants of concern.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.C.L. and R.S.B. hold patents on norovirus vaccine design and ongoing collaborations with Vaxart, Takeda Vaccines, HilleVax, and BioNTech that are unrelated and do not pose conflicts of interest with this report. R.S.B. is a member of the advisory committee for Vaxart and Invivyd.
Figures



References
-
- Liu L., Oza S., Hogan D., Chu Y., Perin J., Zhu J., Lawn J.E., Cousens S., Mathers C., Black R.E. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/s0140-6736(16)31593-8. - DOI - PMC - PubMed
-
- Liu L., Johnson H.L., Cousens S., Perin J., Scott S., Lawn J.E., Rudan I., Campbell H., Cibulskis R., Li M., et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/s0140-6736(12)60560-1. - DOI - PubMed
-
- Becker-Dreps S., Bucardo F., Vilchez S., Zambrana L.E., Liu L., Weber D.J., Peña R., Barclay L., Vinjé J., Hudgens M.G., et al. Etiology of childhood diarrhea after rotavirus vaccine introduction: a prospective, population-based study in Nicaragua. Pediatr. Infect. Dis. J. 2014;33:1156–1163. doi: 10.1097/INF.0000000000000427. - DOI - PMC - PubMed
-
- Koo H.L., Neill F.H., Estes M.K., Munoz F.M., Cameron A., DuPont H.L., Atmar R.L. Noroviruses: the most common pediatric viral enteric pathogen at a large university hospital after introduction of rotavirus vaccination. J. Pediatric Infect. Dis. Soc. 2013;2:57–60. doi: 10.1093/jpids/pis070. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

