Occupation and SARS-CoV-2 seroprevalence studies: a systematic review
- PMID: 36854599
- PMCID: PMC9979591
- DOI: 10.1136/bmjopen-2022-063771
Occupation and SARS-CoV-2 seroprevalence studies: a systematic review
Abstract
Objective: To describe and synthesise studies of SARS-CoV-2 seroprevalence by occupation prior to the widespread vaccine roll-out.
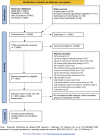
Methods: We identified studies of occupational seroprevalence from a living systematic review (PROSPERO CRD42020183634). Electronic databases, grey literature and news media were searched for studies published during January-December 2020. Seroprevalence estimates and a free-text description of the occupation were extracted and classified according to the Standard Occupational Classification (SOC) 2010 system using a machine-learning algorithm. Due to heterogeneity, results were synthesised narratively.
Results: We identified 196 studies including 591 940 participants from 38 countries. Most studies (n=162; 83%) were conducted locally versus regionally or nationally. Sample sizes were generally small (median=220 participants per occupation) and 135 studies (69%) were at a high risk of bias. One or more estimates were available for 21/23 major SOC occupation groups, but over half of the estimates identified (n=359/600) were for healthcare-related occupations. 'Personal Care and Service Occupations' (median 22% (IQR 9-28%); n=14) had the highest median seroprevalence.
Conclusions: Many seroprevalence studies covering a broad range of occupations were published in the first year of the pandemic. Results suggest considerable differences in seroprevalence between occupations, although few large, high-quality studies were done. Well-designed studies are required to improve our understanding of the occupational risk of SARS-CoV-2 and should be considered as an element of pandemic preparedness for future respiratory pathogens.
Keywords: COVID-19; occupational & industrial medicine; public health.
© World Health Organization 2023. Licensee BMJ.
Conflict of interest statement
Competing interests: RA was previously a Technical Consultant for the Bill and Melinda Gates Foundation Strategic Investment Fund, is a minority shareholder of Alethea Medical and was a former Senior Policy Advisor at Health Canada. Each of these relationships is unrelated to the present work. JP reports grants to his institution from MedImmune, Sanofi Pasteur, Merck and AbbVie, and personal fees for lectures from AbbVie and Astra-Zeneca, all outside of the submitted work. MPC reports grants from McGill Interdisciplinary Initiative in Infection and Immunity, grants from Canadian Institutes of Health Research, during the conduct of the study; personal fees from GEn1E Lifesciences, personal fees from nplex biosciences, personal fees from Kanvas biosciences, personal fees from AstraZeneca, non-financial support from Cidara therapeutics, non-financial support from Scynexis, non-financial support from Amplyx Pharmaceutics, outside the submitted work. In addition, MPC has a patent for methods detecting tissue damage, graft versus host disease, and infections using cell-free DNA profiling pending, a patent for methods assessing the severity and progression of SARS-CoV-2 infections using cell-free DNA pending, a patent for rapid identification of antimicrobial resistance and other microbial phenotypes using highly multiplexed fluorescence in situ hybridisation pending, and a patent highly multiplexed detection of gene expression with hybridisation chain reaction pending, all outside the submitted work.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
