Immunogenicity and protection of a variant nanoparticle vaccine that confers broad neutralization against SARS-CoV-2 variants
- PMID: 36854666
- PMCID: PMC9972327
- DOI: 10.1038/s41467-022-35606-6
Immunogenicity and protection of a variant nanoparticle vaccine that confers broad neutralization against SARS-CoV-2 variants
Abstract
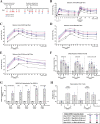
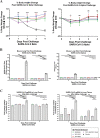
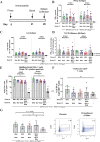
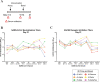
SARS-CoV-2 variants have emerged with elevated transmission and a higher risk of infection for vaccinated individuals. We demonstrate that a recombinant prefusion-stabilized spike (rS) protein vaccine based on Beta/B.1.351 (rS-Beta) produces a robust anamnestic response in baboons against SARS-CoV-2 variants when given as a booster one year after immunization with NVX-CoV2373. Additionally, rS-Beta is highly immunogenic in mice and produces neutralizing antibodies against WA1/2020, Beta/B.1.351, and Omicron/BA.1. Mice vaccinated with two doses of Novavax prototype NVX-CoV2373 (rS-WU1) or rS-Beta alone, in combination, or heterologous prime-boost, are protected from challenge. Virus titer is undetectable in lungs in all vaccinated mice, and Th1-skewed cellular responses are observed. We tested sera from a panel of variant spike protein vaccines and find broad neutralization and inhibition of spike:ACE2 binding from the rS-Beta and rS-Delta vaccines against a variety of variants including Omicron. This study demonstrates that rS-Beta vaccine alone or in combination with rS-WU1 induces antibody-and cell-mediated responses that are protective against challenge with SARS-CoV-2 variants and offers broader neutralizing capacity than a rS-WU1 prime/boost regimen alone. Together, these nonhuman primate and murine data suggest a Beta variant booster dose could elicit a broad immune response to fight new and future SARS-CoV-2 variants.
© 2023. The Author(s).
Conflict of interest statement
N.P., B.Z., S.M., H.Z., A.D.P., J.-H.T., A.R., M.G.-X., M.M., A.M.G., M.J.M., G.M.G., and G.S. are employees of Novavax. J.L., R.M.J., M.E.M., R.E.H., S.M.W., H.H., and M.F. were supported in part by funds from Novavax. M.F. is on the Scientific Advisory Board of Aikido Pharma which has no role in this research. The remaining authors declare no competing interests.
Figures


References
-
- Bermingham A, et al. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Eur. Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2012;17:20290. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

