Cell surface protein aggregation triggers endocytosis to maintain plasma membrane proteostasis
- PMID: 36854675
- PMCID: PMC9974993
- DOI: 10.1038/s41467-023-36496-y
Cell surface protein aggregation triggers endocytosis to maintain plasma membrane proteostasis
Abstract
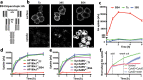
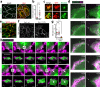
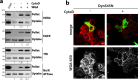
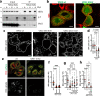
The ability of cells to manage consequences of exogenous proteotoxicity is key to cellular homeostasis. While a plethora of well-characterised machinery aids intracellular proteostasis, mechanisms involved in the response to denaturation of extracellular proteins remain elusive. Here we show that aggregation of protein ectodomains triggers their endocytosis via a macroendocytic route, and subsequent lysosomal degradation. Using ERBB2/HER2-specific antibodies we reveal that their cross-linking ability triggers specific and fast endocytosis of the receptor, independent of clathrin and dynamin. Upon aggregation, canonical clathrin-dependent cargoes are redirected into the aggregation-dependent endocytosis (ADE) pathway. ADE is an actin-driven process, which morphologically resembles macropinocytosis. Physical and chemical stress-induced aggregation of surface proteins also triggers ADE, facilitating their degradation in the lysosome. This study pinpoints aggregation of extracellular domains as a trigger for rapid uptake and lysosomal clearance which besides its proteostatic function has potential implications for the uptake of pathological protein aggregates and antibody-based therapies.
© 2023. The Author(s).
Conflict of interest statement
A.B. and J.D. are employees of the AstraZeneca group of companies and have stocks/stock options in AstraZeneca. The remaining authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

