TGFBI remodels adipose metabolism by regulating the Notch-1 signaling pathway
- PMID: 36854775
- PMCID: PMC10073093
- DOI: 10.1038/s12276-023-00947-9
TGFBI remodels adipose metabolism by regulating the Notch-1 signaling pathway
Abstract
Extracellular matrix proteins are associated with metabolically healthy adipose tissue and regulate inflammation, fibrosis, angiogenesis, and subsequent metabolic deterioration. In this study, we demonstrated that transforming growth factor-beta (TGFBI), an extracellular matrix (ECM) component, plays an important role in adipose metabolism and browning during high-fat diet-induced obesity. TGFBI KO mice were resistant to adipose tissue hypertrophy, liver steatosis, and insulin resistance. Furthermore, adipose tissue from TGFBI KO mice contained a large population of CD11b+ and CD206+ M2 macrophages, which possibly control adipokine secretion through paracrine mechanisms. Mechanistically, we showed that inhibiting TGFBI-stimulated release of adipsin by Notch-1-dependent signaling resulted in adipocyte browning. TGFBI was physiologically bound to Notch-1 and stimulated its activation in adipocytes. Our findings revealed a novel protective effect of TGFBI deficiency in obesity that is realized via the activation of the Notch-1 signaling pathway.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
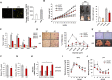




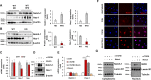

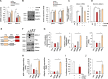
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

