Individual hematotoxicity prediction of further chemotherapy cycles by dynamic mathematical models in patients with gastrointestinal tumors
- PMID: 36854800
- PMCID: PMC10374676
- DOI: 10.1007/s00432-023-04601-9
Individual hematotoxicity prediction of further chemotherapy cycles by dynamic mathematical models in patients with gastrointestinal tumors
Abstract
Purpose: Hematotoxicity is a common side-effect of cytotoxic gastrointestinal (GI) cancer therapies. An unsolved problem is to predict the individual risk therefore to decide on treatment adaptions. We applied an established biomathematical prediction model and primarily evaluated its predictive value in patients undergoing chemotherapy for GI cancers in curative intent.
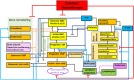
Methods: In a prospective, observational multicenter study on patients with gastro-esophageal or pancreatic cancer (n = 28) receiving myelosuppressive adjuvant or neoadjuvant chemotherapy (FLO(T) or FOLFIRINOX), individual model parameters were learned based on patients' observed laboratory values during the first chemotherapy cycle and further external data resources. Grades of hematotoxicity of subsequent cycles were predicted by model simulation and compared with observed data.
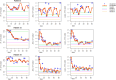
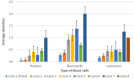
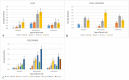
Results: The most common high-grade hematological toxicity was neutropenia [19/28 patients (68%)]. For the FLO(T) regimen, individual grades of thrombocytopenia and leukopenia could be well predicted for cycles 2-4, as well as grades of neutropenia for cycle 2. Prediction accuracy for neutropenia in the third and fourth cycle differed by one toxicity grade on average. For the FOLFIRINOX-regimen, thrombocytopenia predictions showed a maximum deviation of one toxicity grade up to the end of therapy (8 cycles). Deviations of predictions were less than one degree on average up to cycle 4 for neutropenia, and up to cycle 6 for leukopenia.
Conclusion: The biomathematical model showed excellent short-term and decent long-term prediction performance for all relevant hematological side effects associated with FLO(T)/FOLFIRINOX. Clinical utility of this precision-medicine approach needs to be further investigated in a larger cohort.
Keywords: Biomathematical model; Cytotoxic chemotherapy; Gastrointestinal cancer; Hematotoxicity; Pharmacodynamics; Precision medicine.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The authors have no competing interests to declare.
Figures
References
-
- Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD, FLOT4-AIO Investigators (2019) Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. In the Lancet 393(10184):1948–1957. 10.1016/S0140-6736(18)32557-1 - PubMed
-
- Arshad U, Ploylearmsaeng SA, Karlsson MO, Doroshyenko O, Langer D, Schömig E, Kunze S, Güner SA, Skripnichenko R, Ullah S, Jaehde U, Fuhr U, Jetter A, Taubert M (2020) Prediction of exposure-driven myelotoxicity of continuous infusion 5-fluorouracil by a semi-physiological pharmacokinetic-pharmacodynamic model in gastrointestinal cancer patients. Cancer Chemother Pharmacol 85(4):711–722. 10.1007/s00280-019-04028-5 - PMC - PubMed
-
- Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events (Ed.) (2017) Common terminology criteria for adverse events v5.0 (CTCAE). Available online at https://ctep.cancer.gov
-
- Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O’Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB (2018) FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. New England J Med 379(25):2395–2406. 10.1056/NEJMoa1809775 - PubMed
-
- Delord JP, Umlil A, Guimbaud R, Grégoire N, Lafont T, Canal P, Bugat R, Chatelut E (2003) Population pharmacokinetics of oxaliplatin. Cancer Chemother Pharmacol 51(2):127–131. 10.1007/s00280-002-0550-3 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

