SARS-CoV-2-reactive antibody waning, booster effect and breakthrough SARS-CoV-2 infection in hematopoietic stem cell transplant and cell therapy recipients at one year after vaccination
- PMID: 36854892
- PMCID: PMC9974060
- DOI: 10.1038/s41409-023-01946-0
SARS-CoV-2-reactive antibody waning, booster effect and breakthrough SARS-CoV-2 infection in hematopoietic stem cell transplant and cell therapy recipients at one year after vaccination
Abstract
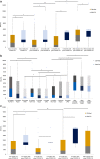
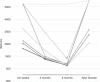
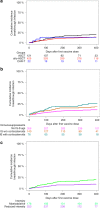
The kinetics of SARS-CoV-2 reactive IgG antibodies after full vaccination and booster in allogeneic and autologous stem cell transplantation (allo-HSCT, ASCT) and chimeric antigen receptor T-cell therapy (CAR-T) are of utmost importance for estimating risk of infection. A prospective multicenter registry-based cohort study, conducted from December 2020 to July 2022 was used to analyze antibody waning over time, booster effect and the relationship of antibody response and breakthrough infection in 572 recipients (429 allo-HSCT, 121 ASCT and 22 CAR-T cell therapy). A significant decline in antibody titers was observed at 3 and 6 months after full vaccination in recipients without pre-vaccine SARS-CoV-2 infection, whereas recipients infected prior to vaccination showed higher and stable antibody titers over time. In poor responders, a booster dose was able to increase antibody titers in 83% of allo-HSCT and 58% of ASCT recipients but not in CART-T cell recipients [0%] (p < 0.01). One-year cumulative incidence of breakthrough infection was 15%, similar among cell therapy procedures. Immunosuppressive drugs at the time of vaccination [hazard ratio (HR) 1.81, p = 0.0028] and reduced intensity conditioning (HR 0.49, p = 0.011) were identified as the only conditions associated with different risk of breakthrough infection in allo-HSCT recipients. Antibody titers were associated with breakthrough infection and disease severity. No death was observed among the 72 breakthrough infections. Antibody level decay after the first two vaccine doses was common except in recipients with pre-vaccination SARS-CoV-2 infection. Poorly responding allo-HSCT recipients showed a response advantage with the booster as compared to ASCT and, especially, the null response found in CAR-T cell recipients. Antibody titers were positively correlated with the risk of breakthrough SARS-CoV-2 infection which was mainly driven by the immunosuppression status.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Spanjaart AM, Ljungman P, de La Camara R, Tridello G, Ortiz-Maldonado V, Urbano-Ispizua A, et al. Poor outcome of patients with COVID-19 after CAR T-cell therapy for B-cell malignancies: results of a multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party and the European Hematology Association (EHA) Lymphoma Group. Leukemia. 2021;35:3585–8. doi: 10.1038/s41375-021-01466-0. - DOI - PMC - PubMed
-
- Piñana JL, López-Corral L, Martino R, Montoro J, Vazquez L, Pérez A, et al. SARS-CoV-2-reactive antibody detection after SARS-CoV-2 vaccination in hematopoietic stem cell transplant recipients: Prospective survey from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group. Am J Hematol. 2022;97:30–42. doi: 10.1002/ajh.26385. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

