Matrix is everywhere: extracellular DNA is a link between biofilm and mineralization in Bacillus cereus planktonic lifestyle
- PMID: 36854956
- PMCID: PMC9975174
- DOI: 10.1038/s41522-023-00377-5
Matrix is everywhere: extracellular DNA is a link between biofilm and mineralization in Bacillus cereus planktonic lifestyle
Abstract
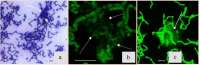
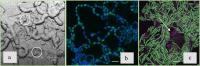
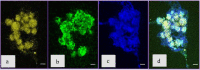
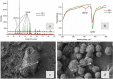
To date, the mechanisms of biomineralization induced by bacterial cells in the context of biofilm formation remain the subject of intensive studies. In this study, we analyzed the influence of the medium components on the induction of CaCO3 precipitation by the Bacillus cereus cells and composition of the extracellular matrix (ECM) formed in the submerged culture. While the accumulation of extracellular polysaccharides and amyloids appeared to be independent of the presence of calcium and urea during the growth, the accumulation of extracellular DNA (eDNA), as well as precipitation of calcium carbonate, required the presence of both ingredients in the medium. Removal of eDNA, which was sensitive to treatment by DNase, did not affect other matrix components but resulted in disruption of cell network formation and a sixfold decrease in the precipitate yield. An experiment with a cell-free system confirmed the acceleration of mineral formation after the addition of exogenous salmon sperm DNA. The observed pathway for the formation of CaCO3 minerals in B. cereus planktonic culture included a production of exopolysaccharides and negatively charged eDNA lattice promoting local Ca2+ supersaturation, which, together with an increase in the concentration of carbonate ions due to pH rise, resulted in the formation of an insoluble precipitate of calcium carbonate. Precipitation of amorphous CaCO3 on eDNA matrix was followed by crystal formation via the ACC-vaterite-calcite/aragonite pathway and further formation of larger mineral aggregates in complex with extracellular polymeric substances. Taken together, our data showed that DNA in extracellular matrix is an essential factor for triggering the biomineralization in B. cereus planktonic culture.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- le Métayer-Levrel G, Castanier S, Orial G, Loubière J-F, Perthuisot J-P. Applications of bacterial carbonatogenesis to the protection and regeneration of limestones in buildings and historic patrimony. Sediment Geol. 1999;126:25–34. doi: 10.1016/S0037-0738(99)00029-9. - DOI
-
- de Muynck W, de Belie N, Verstraete W. Microbial carbonate precipitation in construction materials: a review. Ecol. Eng. 2010;36:118–136. doi: 10.1016/j.ecoleng.2009.02.006. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

