Efficacy and Safety of Baricitinib in Patients with Severe Alopecia Areata over 52 Weeks of Continuous Therapy in Two Phase III Trials (BRAVE-AA1 and BRAVE-AA2)
- PMID: 36855020
- PMCID: PMC9974384
- DOI: 10.1007/s40257-023-00764-w
Efficacy and Safety of Baricitinib in Patients with Severe Alopecia Areata over 52 Weeks of Continuous Therapy in Two Phase III Trials (BRAVE-AA1 and BRAVE-AA2)
Abstract
Background: The oral Janus kinase (JAK) inhibitor baricitinib has demonstrated efficacy for severe alopecia areata (AA) over 36 weeks. There are limited data on the longer-term treatment of AA.
Objective: The aim of this study was to evaluate the efficacy and safety of baricitinib for AA in adults with ≥50% scalp hair loss through 52 weeks of continuous therapy in two phase III trials (BRAVE-AA1 and BRAVE-AA2).
Methods: Patients randomized to baricitinib at baseline in BRAVE-AA1 (N = 465) and BRAVE-AA2 (N = 390) retained their treatment allocation through Week 52. Efficacy outcomes included the proportion of patients achieving a Severity of Alopecia Tool (SALT) score ≤ 20 (≤ 20% scalp hair loss). Data were censored after permanent treatment discontinuation or if collected remotely due to the coronavirus disease 2019 (COVID-19) pandemic.
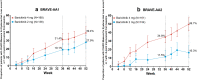
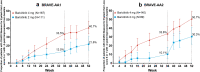
Results: Response rates for hair regrowth increased over the 52-week period. Of patients treated with baricitinib 4 mg and 2 mg, respectively, 40.9% and 21.2% in BRAVE-AA1 and 36.8% and 24.4% in BRAVE-AA2 achieved a SALT score ≤ 20 at Week 52. The most frequent treatment-emergent adverse events included upper respiratory tract infection, headache, nasopharyngitis, acne, urinary tract infection, creatine phosphokinase elevation, and COVID-19 infection.
Limitation: There were no comparisons with placebo.
Conclusion: Efficacy of baricitinib for adults with severe AA continuously improved over 52 weeks, indicating that long-term treatment may be necessary to observe maximum clinical benefit. There were no new safety signals.
Clinicaltrials registration: ClinicalTrials.gov NCT03570749 and NCT03899259. Efficacy and Safety of Baricitinib in Patients with Severe Alopecia Areata: Week-52 Results from BRAVE-AA1 and BRAVE-AA2.
Plain language summary
Alopecia areata (AA) is an autoimmune disease that causes patchy hair loss on the scalp, face, and body. Baricitinib is a Janus kinase inhibitor that is approved to treat AA in several countries, based on results from two studies, BRAVE-AA1 and BRAVE-AA2. In these studies, adults with at least 50% scalp hair loss were treated with baricitinib for 36 weeks. Long-term therapy is important in AA, and hair regrowth can take longer in some patients with severe disease. Therefore, we assessed outcomes from a longer course of therapy. In this study, we report the results after 52 weeks of continuous treatment with baricitinib 4 mg or 2 mg in 465 patients in BRAVE-AA1 and 390 patients BRAVE-AA2. The goal was to reduce scalp hair loss to 20% or less by Week 52. In BRAVE-AA1, 40.9% of patients who took baricitinib 4 mg and 21.2% of patients who took baricitinib 2 mg had 20% or less missing scalp hair by Week 52. Similarly, in BRAVE-AA2, 36.8% of patients who took baricitinib 4 mg and 24.4% of patients who took baricitinib 2 mg had 20% or less missing scalp hair by Week 52. The most common adverse effects that were reported during the study period were upper respiratory tract infection, headache, nasopharyngitis, acne, urinary tract infection, creatine phosphokinase elevation, and coronavirus disease 2019 (COVID-19) infection. The results of longer-term treatment indicate that hair regrowth continues to improve without any new safety concerns for adults with severe AA taking baricitinib.
© 2023. The Author(s).
Conflict of interest statement
Ohsang Kwon reports grants from Lilly during the conduct of the study and grants from Pfizer, Amorepacific Corporation and Juvic Co., and personal fees from Yuhan Pharmaceuticals outside the submitted work. Maryanne M. Senna has served on scientific advisory boards for Lilly, Pfizer, Concert, Arena, Follica, Deciphera, Cassiopea; as a speaker for Lilly & Pfizer; and as principal investigator (PI) for Lilly and Concert. Rodney Sinclair is a member of the Lilly advisory board and was PI in the Lilly-sponsored BRAVE clinical trials. Taisuke Ito has served on advisory boards and/or is a consultant and/or clinical trial investigator for Bristol-Myers Squibb, Lilly, Pfizer, Regeneron, and Maruho Co Ltd. Brett King has served on advisory boards and/or is a consultant and/or clinical trial investigator for AbbVie, AltruBio Inc., Almirall, AnaptysBio, Arena, Bioniz Therapeutics, Bristol-Myers Squibb, Concert, Horizon Therapeutics, Lilly, Incyte, LEO Pharma, Otsuka/Visterra Inc., Pfizer, Regeneron, Sanofi Genzyme, TWi Biotechnology Inc., and Viela Bio. He is on speaker bureaus for AbbVie, Incyte, Lilly, Pfizer, Regeneron, and Sanofi Genzyme. Yves Dutronc, Guanglei Yu, Chiara Chiasserini, Jill McCollam, and Wen-Shuo Wu are employees and shareholders of Lilly. Chen-Yen Lin was an employee of Lilly during the period the studies were conducted.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

