Treatment of spinal muscular atrophy with Onasemnogene Abeparvovec in Switzerland: a prospective observational case series study
- PMID: 36855136
- PMCID: PMC9971686
- DOI: 10.1186/s12883-023-03133-6
Treatment of spinal muscular atrophy with Onasemnogene Abeparvovec in Switzerland: a prospective observational case series study
Abstract
Background: Spinal muscular atrophy (SMA) is a rare neuromuscular disorder leading to early death in the majority of affected individuals without treatment. Recently, targeted treatment approaches including Onasemnogene Abeparvovec (OA) were introduced. This study describes the first real-world experience with OA in Switzerland.
Methods: Prospective observational case series study using data collected within the Swiss Registry for Neuromuscular Disorders from SMA patients treated with OA. Development of motor, bulbar and respiratory function, appearance of scoliosis, and safety data (platelet count, liver function, and cardiotoxicity) were analyzed.
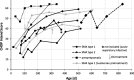
Results: Nine individuals were treated with OA and followed for 383 ± 126 days: six SMA type 1 (of which two with nusinersen pretreatment), one SMA type 2, and two pre-symptomatic individuals. In SMA type 1, CHOP Intend score increased by 28.1 from a mean score of 20.5 ± 7.6 at baseline. At end of follow-up, 50% of SMA type 1 patients required nutritional support and 17% night-time ventilation; 67% developed scoliosis. The SMA type 2 patient and two pre-symptomatically treated individuals reached maximum CHOP Intend scores. No patient required adaptation of the concomitant prednisolone treatment, although transient decrease of platelet count and increase of transaminases were observed in all patients. Troponin-T was elevated prior to OA treatment in 100% and showed fluctuations in 57% thereafter.
Conclusions: OA is a potent treatment for SMA leading to significant motor function improvements. However, the need for respiratory and especially nutritional support as well as the development of scoliosis must be thoroughly evaluated in SMA type 1 patients even in the short term after OA treatment.
Keywords: Gene therapy; Onasemnogene Abeparvovec; Spinal muscular atrophy; Switzerland; Zolgensma.
© 2023. The Author(s).
Conflict of interest statement
GMS served on advisory boards of AveXis, Biogen, Novartis Gene Therapies and Roche.
OH served on advisory boards of Biogen and Novartis Gene Therapies; he received speaker’s honoraria from Biogen. DJ served on advisory boards of AveXis, Biogen, Novartis Gene Therapies and Roche; he received speaker’s honoraria from Biogen and Roche, and a research grant from AveXis, Biogen and Roche. AT is employee of the Swiss-Reg-NMD.
EG has no conflict of interest to report. AK served on advisory boards of AveXis, Biogen, Novartis Gene Therapies, Roche, Sarepta and Pfizer; she received speaker’s honoraria from Roche, Biogen, Pfizer and Novartis Gene Therapies.
The Swiss-Reg-NMD is funded by ‘Schweizerische Muskelgesellschaft’, ‘Association Suisse Romande Intervenant contre les Maladies neuro-Musculaires’, ‘Associazione Malattie Genetiche Rare Svizzera italiana’, ‘SMA Schweiz’, ‘Duchenne Schweiz’ ‘Schweizerische Stiftung für die Erforschung der Muskelkrankheiten’ FSRMM; and receives unconditional and research grants from PTC Therapeutics lnternational, Sarepta International, Pfizer Switzerland, AveXis EU Limited, Roche Pharma Schweiz, Novartis Gene Therapies, and Biogen Switzerland AG. None of the funders was involved in the study design, data collection, analysis, interpretation of data or writing the article.
Figures
References
-
- Prior TW, Leach ME, Finanger E. Spinal muscular atrophy - PubMed n.d. https://pubmed.ncbi.nlm.nih.gov/20301526/ (Accessed 25 June 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

