Monocyte-derived cells invade brain parenchyma and amyloid plaques in human Alzheimer's disease hippocampus
- PMID: 36855152
- PMCID: PMC9976401
- DOI: 10.1186/s40478-023-01530-z
Monocyte-derived cells invade brain parenchyma and amyloid plaques in human Alzheimer's disease hippocampus
Abstract
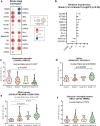
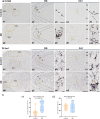
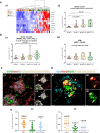
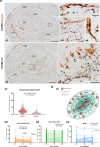
Microglia are brain-resident myeloid cells and play a major role in the innate immune responses of the CNS and the pathogenesis of Alzheimer's disease (AD). However, the contribution of nonparenchymal or brain-infiltrated myeloid cells to disease progression remains to be demonstrated. Here, we show that monocyte-derived cells (MDC) invade brain parenchyma in advanced stages of AD continuum using transcriptional analysis and immunohistochemical characterization in post-mortem human hippocampus. Our findings demonstrated that a high proportion (60%) of demented Braak V-VI individuals was associated with up-regulation of genes rarely expressed by microglial cells and abundant in monocytes, among which stands the membrane-bound scavenger receptor for haptoglobin/hemoglobin complexes or Cd163. These Cd163-positive MDC invaded the hippocampal parenchyma, acquired a microglial-like morphology, and were located in close proximity to blood vessels. Moreover, and most interesting, these invading monocytes infiltrated the nearby amyloid plaques contributing to plaque-associated myeloid cell heterogeneity. However, in aged-matched control individuals with hippocampal amyloid pathology, no signs of MDC brain infiltration or plaque invasion were found. The previously reported microglial degeneration/dysfunction in AD hippocampus could be a key pathological factor inducing MDC recruitment. Our data suggest a clear association between MDC infiltration and endothelial activation which in turn may contribute to damage of the blood brain barrier integrity. The recruitment of monocytes could be a consequence rather than the cause of the severity of the disease. Whether monocyte infiltration is beneficial or detrimental to AD pathology remains to be fully elucidated. These findings open the opportunity to design targeted therapies, not only for microglia but also for the peripheral immune cell population to modulate amyloid pathology and provide a better understanding of the immunological mechanisms underlying the progression of AD.
Keywords: Alzheimer’s disease; Amyloid plaques; Brain infiltration; Human hippocampus; Microglia; Myeloid cells.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

References
-
- Baruch K, Deczkowska A, Rosenzweig N, Tsitsou-Kampeli A, Sharif AM, Matcovitch-Natan O, Kertser A, David E, Amit I, Schwartz M. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat Med. 2016;22:135–137. doi: 10.1038/NM.4022. - DOI - PubMed
-
- Böttcher C, Schlickeiser S, Sneeboer MAM, Kunkel D, Knop A, Paza E, Fidzinski P, Kraus L, Snijders GJL, Kahn RS, Schulz AR, Mei HE, Hol EM, Siegmund B, Glauben R, Spruth EJ, de Witte LD, Priller J. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat Neurosci. 2019;22:78–90. doi: 10.1038/S41593-018-0290-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

