Comparative study of extracellular vesicles derived from mesenchymal stem cells and brain endothelial cells attenuating blood-brain barrier permeability via regulating Caveolin-1-dependent ZO-1 and Claudin-5 endocytosis in acute ischemic stroke
- PMID: 36855156
- PMCID: PMC9976550
- DOI: 10.1186/s12951-023-01828-z
Comparative study of extracellular vesicles derived from mesenchymal stem cells and brain endothelial cells attenuating blood-brain barrier permeability via regulating Caveolin-1-dependent ZO-1 and Claudin-5 endocytosis in acute ischemic stroke
Abstract
Background: Blood-brain barrier (BBB) disruption is a major adverse event after ischemic stroke (IS). Caveolin-1 (Cav-1), a scaffolding protein, played multiple roles in BBB permeability after IS, while the pros and cons of Cav-1 on BBB permeability remain controversial. Numerous studies revealed that extracellular vesicles (EVs), especially stem cells derived EVs, exerted therapeutic efficacy on IS; however, the mechanisms of BBB permeability needed to be clearly illustrated. Herein, we compared the protective efficacy on BBB integrity between bone marrow mesenchymal stem cells derived extracellular vesicles (BMSC-EVs) and EVs from brain endothelial cells (BEC-EVs) after acute IS and investigated whether the mechanism was associated with EVs antagonizing Cav-1-dependent tight junction proteins endocytosis.
Methods: BMSC-EVs and BEC-EVs were isolated and characterized by nanoparticle tracking analysis, western blotting, and transmission electron microscope. Oxygen and glucose deprivation (OGD) treated b. End3 cells were utilized to evaluate brain endothelial cell leakage. CCK-8 and TRITC-dextran leakage assays were used to measure cell viability and transwell monolayer permeability. Permanent middle cerebral artery occlusion (pMCAo) model was established, and EVs were intravenously administered in rats. Animal neurological function tests were applied, and microvessels were isolated from the ischemic cortex. BBB leakage and tight junction proteins were analyzed by Evans Blue (EB) staining and western blotting, respectively. Co-IP assay and Cav-1 siRNA/pcDNA 3.1 vector transfection were employed to verify the endocytosis efficacy of Cav-1 on tight junction proteins.
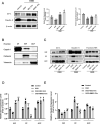
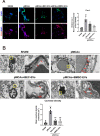
Results: Both kinds of EVs exerted similar efficacies in reducing the cerebral infarction volume and BBB leakage and enhancing the expressions of ZO-1 and Claudin-5 after 24 h pMCAo in rats. At the same time, BMSC-EVs were outstanding in ameliorating neurological function. Simultaneously, both EVs treatments suppressed the highly expressed Cav-1 in OGD-exposed b. End3 cells and ischemic cerebral microvessels, and this efficacy was more prominent after BMSC-EVs administration. Cav-1 knockdown reduced OGD-treated b. End3 cells monolayer permeability and recovered ZO-1 and Claudin-5 expressions, whereas Cav-1 overexpression aggravated permeability and enhanced the colocalization of Cav-1 with ZO-1 and Claudin-5. Furthermore, Cav-1 overexpression partly reversed the lower cell leakage by BMSC-EVs and BEC-EVs administrations in OGD-treated b. End3 cells.
Conclusions: Our results demonstrated that Cav-1 aggravated BBB permeability in acute ischemic stroke, and BMSC-EVs exerted similar antagonistic efficacy to BEC-EVs on Cav-1-dependent ZO-1 and Claudin-5 endocytosis. BMSC-EVs treatment was superior in Cav-1 suppression and neurological function amelioration.
Keywords: Blood–brain barrier; Caveolin-1; Endocytosis; Extracellular vesicles; Ischemic stroke; Tight junction proteins.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929–1935. doi: 10.1016/s0140-6736(14)60584-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

