Global distribution of functionally important CYP2C9 alleles and their inferred metabolic consequences
- PMID: 36855170
- PMCID: PMC9976394
- DOI: 10.1186/s40246-023-00461-z
Global distribution of functionally important CYP2C9 alleles and their inferred metabolic consequences
Abstract
Background: Genetic variability in the cytochrome P450 CYP2C9 constitutes an important predictor for efficacy and safety of various commonly prescribed drugs, including coumarin anticoagulants, phenytoin and multiple non-steroidal anti-inflammatory drugs (NSAIDs). A global map of CYP2C9 variability and its inferred functional consequences has been lacking.
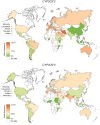
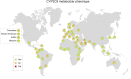
Results: Frequencies of eight functionally relevant CYP2C9 alleles (*2, *3, *5, *6, *8, *11, *13 and *14) were analyzed. In total, 108 original articles were identified that included genotype data from a total of 81,662 unrelated individuals across 70 countries and 40 unique ethnic groups. The results revealed that CYP2C9*2 was most abundant in Europe and the Middle East, whereas CYP2C9*3 was the main reason for reduced CYP2C9 activity across South Asia. Our data show extensive variation within superpopulations with up to tenfold differences between geographically adjacent populations in Malaysia, Thailand and Vietnam. Translation of genetic CYP2C9 variability into functional consequences indicates that up to 40% of patients in Southern Europe and the Middle East might benefit from warfarin and phenytoin dose reductions, while 3% of patients in Southern Europe and Israel are recommended to reduce starting doses of NSAIDs.
Conclusions: This study provides a comprehensive map of the genetic and functional variability of CYP2C9 with high ethnogeographic resolution. The presented data can serve as a useful resource for CYP2C9 allele and phenotype frequencies and might guide the optimization of genotyping strategies, particularly for indigenous and founder populations with distinct genetic profiles.
Keywords: Allele frequency; Metabolizer phenotype; Pharmacogenomics; Precision medicine; Precision public health.
© 2023. The Author(s).
Conflict of interest statement
YZ and VML are co-founders and shareholders of PersoMedix AB. In addition, VML is CEO and shareholder of HepaPredict AB. EE is vice-chair of the Genomic Medicine Sweden Pharmacogenomics work package, supported by grants from The Swedish Innovation Agency. EE also received funding from the Stockholm Region (CIMED). The other authors do not disclose competing interests.
Figures
References
-
- Impicciatore P, Choonara I, Clarkson A, Provasi D, Pandolfini C, Bonati M. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol. 2001;52(1):77–83. doi: 10.1046/j.0306-5251.2001.01407.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

