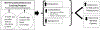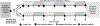The Support, Educate, Empower personalized glaucoma coaching trial design
- PMID: 36855233
- PMCID: PMC10023277
- DOI: 10.1177/17407745221136571
The Support, Educate, Empower personalized glaucoma coaching trial design
Abstract
Background: Glaucoma is a chronic disease that affects 3 million Americans. Glaucoma is most often asymptomatic until very late in its course when treatment is more difficult and extensive peripheral vision loss has already occurred. Taking daily medications can mitigate this vision loss, but at least half of people with glaucoma do not take their prescribed medications regularly. The purpose of this study is to improve glaucoma medication adherence among those with medically treated glaucoma and poor self-reported adherence using the Support, Educate, Empower personalized coaching program.
Methods/design: This study is a two-site randomized controlled trial enrolling 230 participants with poor self-reported glaucoma medication adherence. The trial has two arms, an intervention arm and a control arm. Participants in the intervention arm receive personalized glaucoma education and motivational interviewing-based coaching over 6 months from a trained non-physician interventionist for three in-person sessions with between visit phone calls for check-ins where current adherence level is reported to participants. Participants also can elect to have visual, audio, text or automated phone call medication dose reminders. Participants in the control arm continue usual care with their physician and receive non-personalized glaucoma educational materials via mail in parallel to the three in-person coaching sessions to control for glaucoma knowledge content. All participants receive a medication adherence monitor. The primary outcome is the proportion of prescribed doses taken on schedule during the 6-month period. The secondary outcome is glaucoma related distress. The exploratory outcome is intraocular pressure.
Discussion: The personalized education and motivational-interviewing-based intervention that we are testing is comprehensive in that it addresses the wide range of barriers to adherence that people with glaucoma encounter. Leveraging a custom-built web-based application to generate the personalized content and the motivational-interviewing-based prompts to guide the coaching sessions will make this program both replicable and scalable and can be integrated into clinical care utilizing trained non-physician providers. Although this type of self-management support is not currently reimbursed for glaucoma as it is for diabetes, this trial could help shape future policy change should the intervention be found effective.
Keywords: Glaucoma; health coaching; medication adherence; motivational interviewing; multi-pronged intervention; personalized education; randomized controlled trial; self-management support; tailored education.
Conflict of interest statement
Conflict of Interest: The authors have no proprietary or commercial interest in any of the materials discussed in this article.
Figures
References
-
- Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma(UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet 2015; 385: 1295–1304. - PubMed
-
- Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch Ophthal 2003;121(1):48–56. - PubMed
-
- Bourne RRA, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health 2013; 1: e339–49. - PubMed
-
- World Health Organization. Blindness and Vision Impairment Prevention: Priority Eye Diseases, Glaucoma, https://www.who.int/blindness/causes/priority/en/index6.html. (Accessed 30 May 2022).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous